Preparation method of high-stability carboxylic chloroprene rubber latex
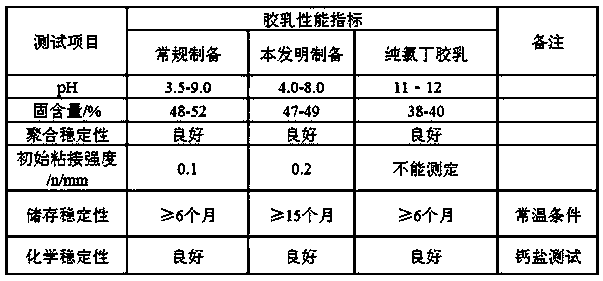
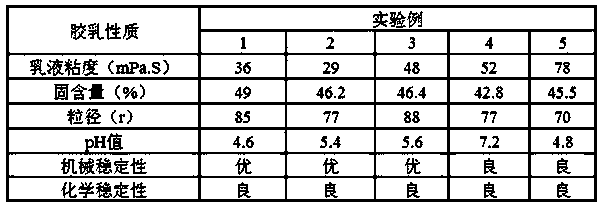
A carboxyl neoprene latex, high stability technology, applied in the direction of carboxyl rubber adhesives, adhesives, adhesive types, etc., can solve the problem of high activity of chloroprene active groups, unfavorable ecological environment and public health, and easy Combustion and other problems, to solve the problem of mechanical stability and chemical stability, improve the poor stability of latex, improve the effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
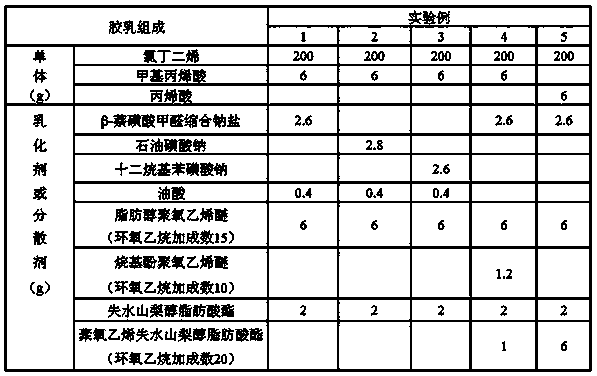
[0024] Embodiment 1: a kind of preparation method of high stability carboxyl neoprene latex, comprises the steps:
[0025] (1) Preparation of oil phase: Dissolve 0.4 parts of chain transfer agent, 0.5 parts of nonionic emulsifier, and 0.2 parts of dispersant in 100 parts of chloroprene to prepare oil phase, wherein the HLB of the nonionic emulsifier is ≥ 9;
[0026] (2) Prepare the water phase: dissolve 1.5 parts of anionic emulsifier and 3 parts of nonionic emulsifier in 100 parts of soft water, and stir evenly;
[0027] (3) Polymerization reaction: Divide the oil phase into 2 parts on average, add 3 parts of the second monomer of unsaturated carboxylic acid to one part, then mix it with the water phase at a ratio of 1:2, and stir at 40°C Emulsify to form a stable emulsion system. After the emulsification is completed, add a free radical initiator and carry out polymerization reaction at 10°C. After 3 hours, add another oil phase dropwise. The dropping time is controlled with...
Embodiment 2
[0032] Embodiment 2: a kind of preparation method of high stability carboxyl neoprene latex, comprises the steps:
[0033] (1) Preparation of oil phase: Dissolve 0.4 parts of chain transfer agent, 1 part of nonionic emulsifier, and 0.2 parts of dispersant in 100 parts of chloroprene to prepare oil phase, wherein the HLB of the nonionic emulsifier is ≥ 9;
[0034] (2) Prepare the water phase: dissolve 1 part of anionic emulsifier and 3 parts of nonionic emulsifier in 100 parts of soft water, and stir evenly;
[0035] (3) Polymerization reaction: Divide the oil phase into 2 parts on average, add 3 parts of the second monomer of unsaturated carboxylic acid to one part, then mix it with the water phase at a ratio of 1:2, and stir at 43 °C Emulsify to form a stable emulsion system. After the emulsification is completed, add a free radical initiator and carry out polymerization reaction at 30°C. After 3 hours, add another oil phase dropwise. The dropping time is controlled within 1 ...
Embodiment 3
[0037] Embodiment 3: a kind of preparation method of high stability carboxyl neoprene latex, comprises the steps:
[0038] (1) Preparation of oil phase: Dissolve 0.4 parts of chain transfer agent, 1 part of nonionic emulsifier, and 1 part of dispersant in 100 parts of chloroprene to prepare oil phase, wherein the HLB of the nonionic emulsifier is ≥ 9;
[0039] (2) Prepare the water phase: dissolve 2 parts of anionic emulsifier and 3 parts of nonionic emulsifier in 100 parts of soft water, and stir evenly;
[0040] (3) Polymerization reaction: Divide the oil phase into 2 parts on average, add 3 parts of the second monomer of unsaturated carboxylic acid to one part, then mix it with the water phase at a ratio of 1:2, and stir at 45 °C Emulsify to form a stable emulsion system. After the emulsification is complete, add a free radical initiator and carry out polymerization reaction at 50°C. After 3 hours, add another oil phase dropwise. The dropping time is controlled within 1 hou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com