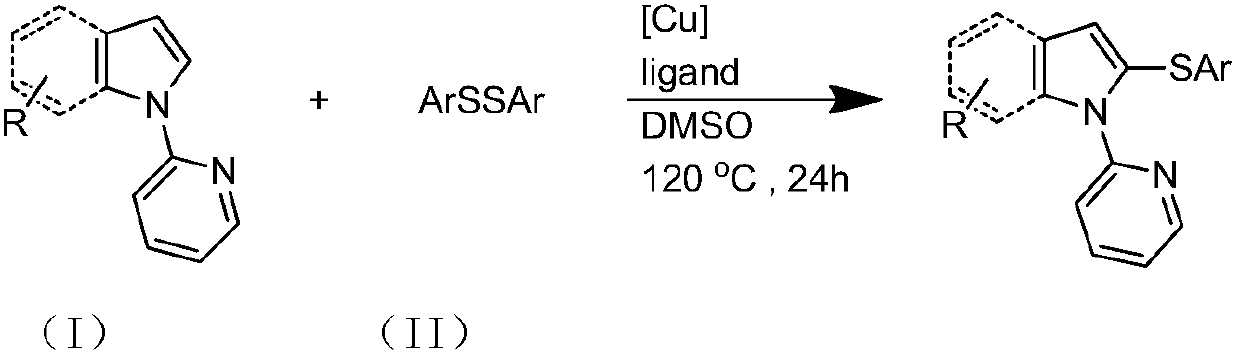
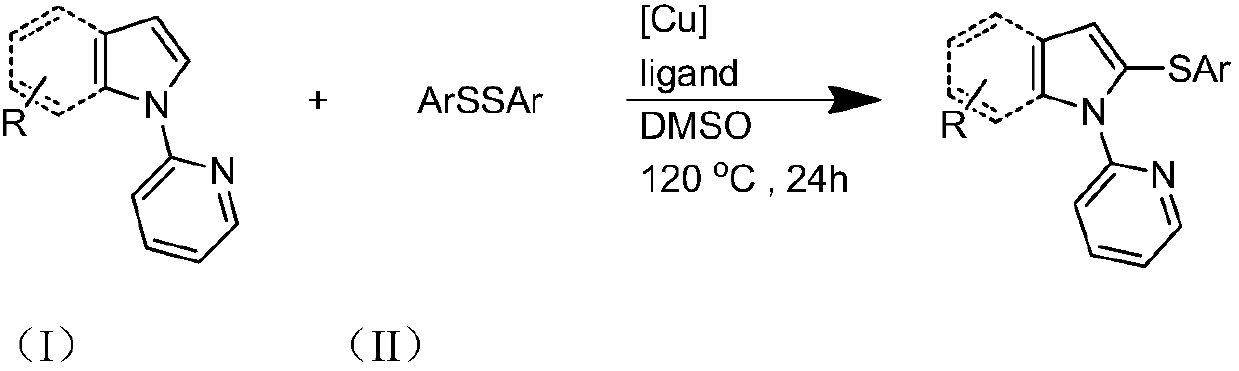
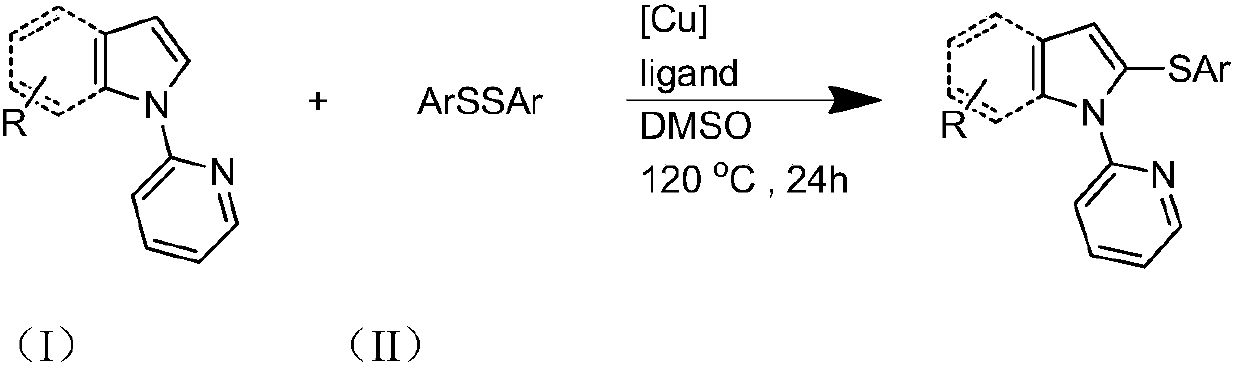
Preparation method and purification method of heterocyclic nitrogen sulfide
A technology of sulfide and nitrogen heterocycle, applied in the field of preparation of nitrogen-containing heterocycle sulfide, can solve problems such as unfavorable industrialization popularization, complex cobalt complex, expensive structure, etc., and achieves cheap synthesis system, simple process flow and cost low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
[0031] Specific Example 1: The substrate is N-pyridylpyrrole and diphenyl disulfide molar ratio is 1:2, the molar percentage of cuprous iodide and N-pyridylpyrrole is 10%, and the ligand is 2-bicyclic Hexylphosphonium-2,4,6-triisopropylbiphenyl, the molar percentage of ligand and N-pyridylpyrrole is 10%, and the reaction temperature is 120°C;
[0032] 28.8 mg (0.2 mmol) N-pyridyl pyrrole, 87.3 mg (0.4 mmol) diphenyl disulfide, 3.8 mg (0.02 mmol) cuprous iodide, 9.6 mg (0.02 mmol) 2-dicyclohexylphosphorus-2 , Add 4,6-triisopropylbiphenyl into the reaction test tube, then add 2mL dimethyl sulfoxide, react at 120°C for 24 hours, cool after the reaction, filter, spin the filtrate, remove the solvent, and use silica gel Column chromatography, petroleum ether washing, TLC detection, combined effluent containing product, rotary evaporator distillation to remove solvent, vacuum drying to obtain light yellow oily liquid N-pyridyl-2,5-diphenylthiopyrrole, yield 81%. 1 HNMR (500MHz, CD...
specific Embodiment 2
[0033] Specific embodiment 2: variable is that the mole percent of cuprous iodide and substrate I is 20mol%;
[0034] 28.8 mg (0.2 mmol) N-pyridyl pyrrole, 87.3 mg (0.4 mmol) diphenyl disulfide, 7.6 mg (0.04 mmol) cuprous iodide, 9.6 mg (0.02 mmol) 2-dicyclohexylphosphorus-2 , Add 4,6-triisopropylbiphenyl into the reaction test tube, then add 2mL dimethyl sulfoxide, react at 120°C for 24 hours, cool after the reaction, filter, spin the filtrate, remove the solvent, and use silica gel Column chromatography, petroleum ether washing, TLC detection, combined effluent containing product, rotary evaporator distillation to remove solvent, vacuum drying to obtain light yellow oily liquid N-pyridyl-2,5-diphenylthiopyrrole, yield 68%.
specific Embodiment 3
[0035] Specific embodiment 3: variable is that the mole percent of cuprous iodide and substrate I is 15mol%;
[0036] 28.8 mg (0.2 mmol) N-pyridyl pyrrole, 87.3 mg (0.4 mmol) diphenyl disulfide, 5.7 mg (0.03 mmol) cuprous iodide, 9.6 mg (0.02 mmol) 2-dicyclohexylphosphorus-2 , Add 4,6-triisopropylbiphenyl into the reaction test tube, then add 2mL dimethyl sulfoxide, react at 120°C for 24 hours, cool after the reaction, filter, spin the filtrate, remove the solvent, and use silica gel Column chromatography, petroleum ether washing, TLC detection, combined effluent containing product, rotary evaporator distillation to remove solvent, vacuum drying to obtain light yellow oily liquid N-pyridyl-2,5-diphenylthiopyrrole, yield 73%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com