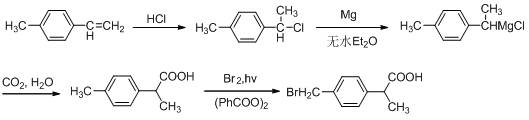
Pipeline preparation method and device for 2-(4-bromomethylphenyl)propionic acid
A technology of bromomethylphenyl and methylphenyl, which is applied in the field of pipeline preparation of 2-propionic acid, can solve the problems of serious environmental protection and safety, increased process cost, and difficulty in separation and extraction, and achieves less three wastes and high atom utilization , good economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
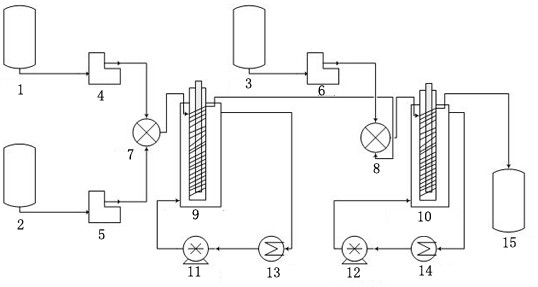
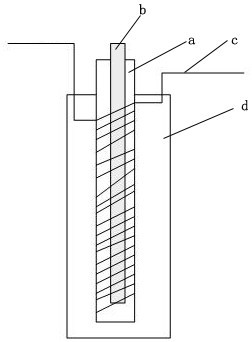
[0055] The structure of the reaction device is as figure 1 , the first tubular photoreactor with a built-in light source 9: the length of the vitreous pipe winding part is 10cm, the inner diameter is 10cm; the inner diameter of the jacket is 20cm; the length of the pipe is 1m, the diameter is 0.5mm, and the pipe material is polytetrafluoroethylene tube; the light source is a 250nm UV lamp with a diameter of 2mm. The second tubular photoreactor 10 with a built-in light source: the length of the winding part of the vitreous pipe is 20 cm, and the inner diameter is 10 cm; the inner diameter of the jacket is 20 cm; the length of the pipe is 30 m, the diameter is 1 mm, and the pipe material is a polytetrafluoroethylene pipe; The light source was a 250 nm UV lamp with a diameter of 2 mm.
[0056] The operation steps are as follows:
[0057] First turn on the light sources of the first tubular photoreactor 9 with a built-in light source and the second tubular photoreactor 10 with ...
Embodiment 2
[0059] The structure of the reaction device is as figure 1 , the first tubular photoreactor 9 with a built-in light source: the length of the winding part of the vitreous pipe is 25cm; the length of the pipe is 50m, and the diameter is 3mm; the light source is a 365nm UV lamp with a diameter of 2mm. The second tubular photoreactor 10 with a built-in light source: the length of the winding part of the vitreous tube is 25 cm; the length of the tube is 50 m, and the diameter is 2 mm; the light source is a 365nm UV lamp with a diameter of 2 mm. Others are the same as embodiment 1.
[0060] The first tubular photoreactor 9 preheating temperature with built-in light source is 60 ℃, and raw material 2-(4-methylphenyl) propionic acid and benzoyl peroxide (consumption is based on 2-(4-methylbenzene) The mass of base) propionic acid is 10%) dissolved in chloroform (solvent consumption is 8mL / g based on the mass of 2-(4-methylphenyl) propionic acid) and stored in the first reservoir 1,...
Embodiment 3
[0062] The structure of the reaction device is as figure 1 , the first tubular photoreactor 9 with a built-in light source: the length of the winding part of the vitreous pipe is 20cm; the length of the pipe is 30m, and the diameter is 1mm; the light source is a 365nm UV lamp with a diameter of 2mm. The second tubular photoreactor 10 with a built-in light source: the length of the winding part of the vitreous tube is 20 cm; the length of the tube is 30 m, and the diameter is 1 mm; the light source is a 365nm UV lamp with a diameter of 2 mm. Others are the same as embodiment 1.
[0063] The first tubular photoreactor 9 preheating temperature with built-in light source is 80 ℃, and raw material 2-(4-methylphenyl) propionic acid and benzoyl peroxide (consumption is based on 2-(4-methylbenzene) The mass of 2-(4-methylphenyl)propionic acid is 5%) dissolved in 1,2-dichloroethane (the amount of solvent is 6mL / g based on the mass of 2-(4-methylphenyl)propionic acid) and stored in No...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com