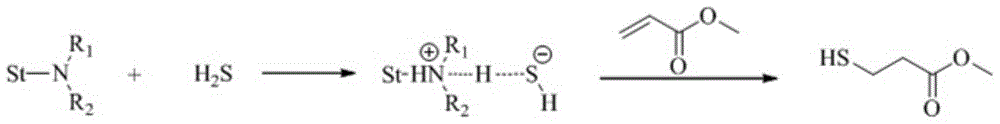
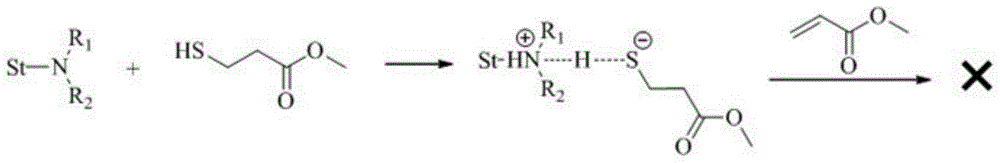
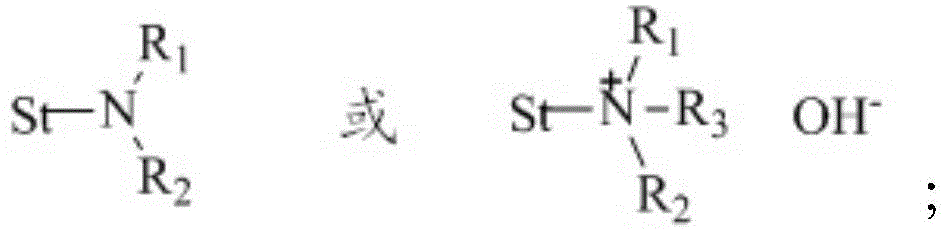A kind of preparation method of thiol compound
A technology for thiol compounds and organic compounds, which is applied in the field of thiol compound preparation, can solve the problems of complicated reaction process, poor catalyst selectivity, increased production cost, etc., and achieves high atomic economic utilization rate, high reaction conversion rate and selectivity. , The effect of easy filtration and recycling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The preparation method of triethylamine starch quaternary ammonium base catalyst is as follows:
[0030] (1) Preparation of cationic etherifying agent:
[0031] Prepared with reference to the method reported by CHILDERS et al. (WO0010987) with slight changes, that is: add 90g of epichlorohydrin and 50mL of dichloromethane into a 250mL three-necked flask, cool to 10°C, and then add 49g of triethylamine (30min) And continue to react for about 5 hours, and check the reaction end point by gas phase; after the reaction of triethylamine is complete, add 30mL of water, stir for 30min, separate the water phase, continue to use dichloromethane to extract the water phase (50mL×3), and the remaining water phase The content of the product is determined by the Kjeldahl method, and the determination method refers to the literature "Wang Xiaojuan et al. Synthesis of dialdehyde starch-aniline Schiff base [J]. Fine Chemical Industry, 2012, 29, 601-605)"; other cationic etherification T...
Embodiment 1
[0041] In a 100mL four-neck flask, add 1g of triethylamine starch quaternary ammonium base catalyst, 30mL of ethanol, stir, feed nitrogen for 30min, then heat up to 60°C, then feed hydrogen sulfide gas (0.8L / h) into the reaction solution, And add dropwise acrylamide solution (5g diluted to 20mL); after a period of reaction, take samples for liquid phase, liquid quality, and gas analysis, until the liquid phase detection shows that the product content does not change within 1h, stop feeding hydrogen sulfide gas , nitrogen, cooled to room temperature, then the reaction solution was filtered, and the filtrate was distilled under reduced pressure to remove ethanol, the resulting solid was added to 50mL water, and 2mol.L -1 NaOH solution to adjust the pH of the solution to about 8. After stirring for 20-30min, the solution was extracted with ethyl acetate (3×20mL), and the remaining aqueous phase was adjusted to pH 3-4 with 2mol.L-1 hydrochloric acid, and then acetic acid was used t...
Embodiment 2
[0043] Add 1.6 g of methylbenzylamine starch catalyst (DS=0.35) and 20 mL of methanol into a 100 mL four-neck flask, stir, feed nitrogen gas for 30 min, and then cool down to 20° C., then feed hydrogen sulfide gas (0.4 L / h), and methyl vinyl ketone solution (5g diluted to 20mL) was added dropwise; after a period of reaction, samples were taken for liquid phase, liquid mass, and gas analysis until the liquid phase detection showed that the product content did not change within 1h. Finally, stop feeding hydrogen sulfide gas and nitrogen gas, then filter the reaction solution, carry out vacuum distillation on the filtrate, and collect products with a boiling point of 75-77°C. The results of liquid phase and liquid mass analysis show that the conversion rate of methyl vinyl ketone can reach 85%, the selectivity of reaction to form methyl mercaptoethyl ketone is 93%, and the yield is 75%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of grafting | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com