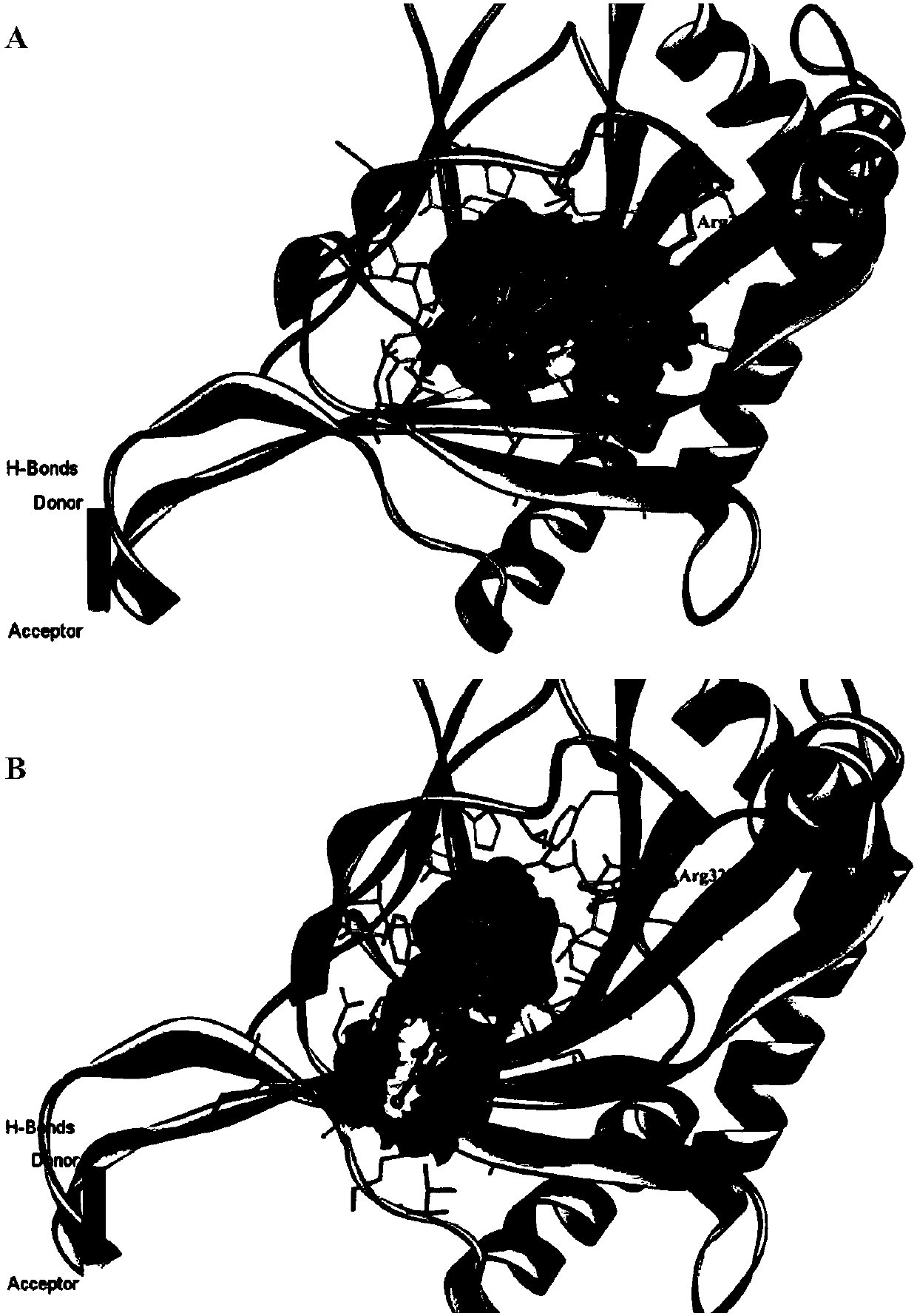
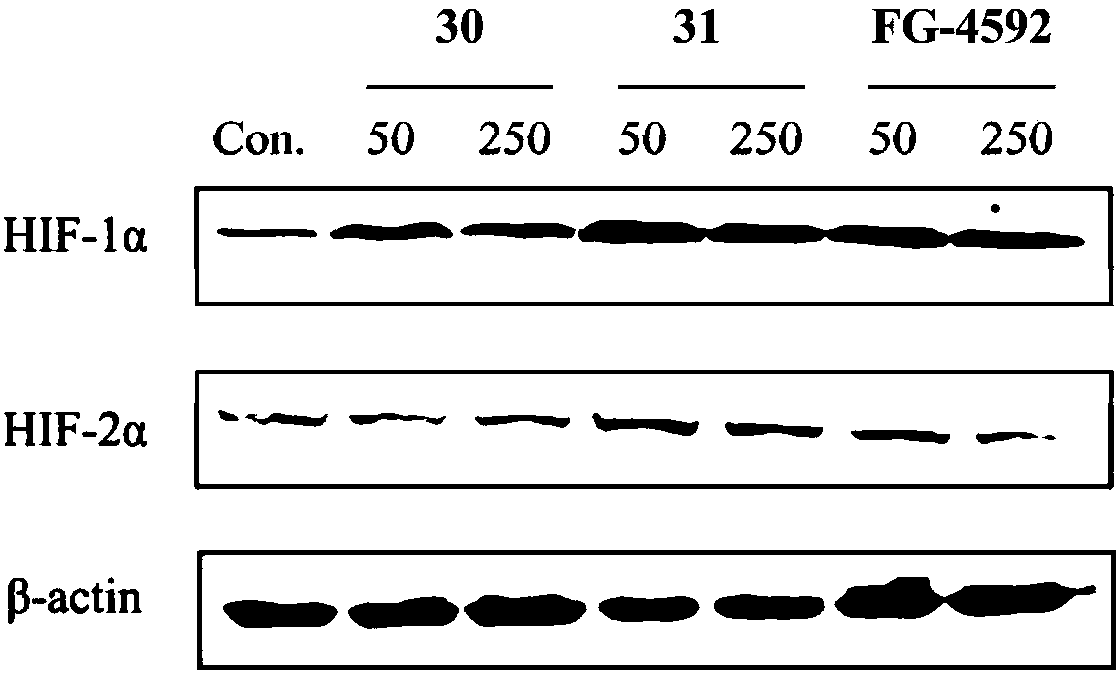
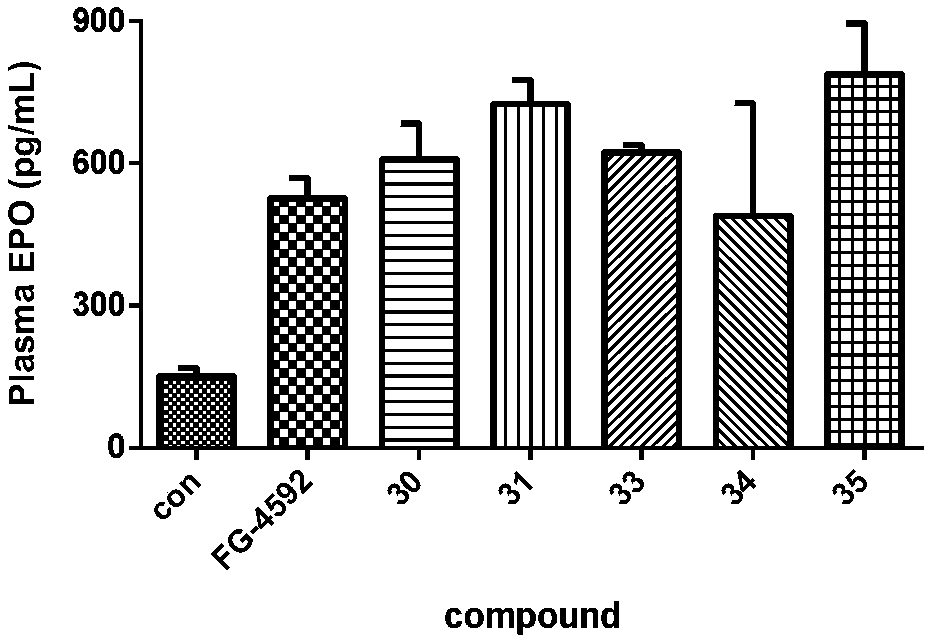
Triazopyridine formylglycine compound, and method and medicinal application thereof
A compound and pharmaceutical technology, which is applied in the field of triazole pyridine glycine compounds, can solve the problems that nutrition therapy is difficult to play a corresponding role, and achieve the effects of promoting EPO production and secretion, increasing EPO production and secretion, and strongly inhibiting activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] N-(5-(1-Benzyl-1H-1,2,3-triazol-4-yl)-3-hydroxy-6-methylpicolyl)glycine
[0059] N-((5-(trimethylsilyl)ethynyl)-3-hydroxy-6-methylpicolyl)glycine methyl ester (200mg, 0.69mmol) was dissolved in 10mL of methanol, and 0.2mL of N,N- Diisopropylethylamine, 20mg cuprous iodide, 1mL TBAF and benzyl azide (110mg, 0.82mmol), conventionally heated to 80°C for 4h or microwaved to 120°C for 10min, the reaction was complete. After the reaction, cuprous iodide was removed by suction filtration, and after distillation under reduced pressure, the crude product was separated and purified by silica gel column chromatography (dichloromethane:methanol=100:1), and the white solid was dissolved in 10 mL of tetrahydrofuran, and 3 mL of 1M hydroxide was added to Lithium solution was heated to 30°C for 2 hours, and the reaction was complete. After the reaction, tetrahydrofuran in the reaction solution was distilled off under reduced pressure, and 3 mmol of dilute hydrochloric acid was added u...
Embodiment 2
[0061] N-(5-(1-Benzyl-1H-1,2,3-triazol-4-yl)-6-chloro-3-hydroxypicolinate)glycine
[0062]N-((5-(trimethylsilyl)ethynyl)-6-chloro-3-hydroxypyridinecarboyl)glycine methyl ester (200mg, 0.69mmol) was dissolved in 10mL methanol, and 0.2mL N,N- Diisopropylethylamine, 20mg cuprous iodide, 1mL TBAF and benzyl azide (110mg, 0.82mmol), conventionally heated to 80°C for 4h or microwaved to 120°C for 10min, the reaction was complete. After the reaction, cuprous iodide was removed by suction filtration, and after distillation under reduced pressure, the crude product was separated and purified by silica gel column chromatography (dichloromethane:methanol=100:1), and the white solid was dissolved in 10 mL of tetrahydrofuran, and 3 mL of 1M hydroxide was added to Lithium solution was heated to 30°C for 2 hours, and the reaction was complete. After the reaction, tetrahydrofuran in the reaction solution was distilled off under reduced pressure, and 3 mmol of dilute hydrochloric acid was add...
Embodiment 3
[0064] N-(5-(1-(1-(4-chlorophenyl)cyclopropyl-1H-1,2,3-triazol-4-yl)-3-hydroxy-6-methylpicolinyl ) Glycine
[0065] N-((5-(trimethylsilyl)ethynyl)-3-hydroxy-6-methylpicolyl)glycine methyl ester (200mg, 0.69mmol) was dissolved in 10mL of methanol, and 0.2mL of N,N- Diisopropylethylamine, 20mg cuprous iodide, 1mL TBAF and 4-chlorophenylcyclopropyl azide (110mg, 0.82mmol), conventionally heated to 80°C for 4h or microwaved to 120°C for 10min, the reaction was complete. After the reaction, cuprous iodide was removed by suction filtration, and after distillation under reduced pressure, the crude product was separated and purified by silica gel column chromatography (dichloromethane:methanol=100:1), and the white solid was dissolved in 10 mL of tetrahydrofuran, and 3 mL of 1M hydroxide was added to Lithium solution was heated to 30°C for 2 hours, and the reaction was complete. After the reaction, tetrahydrofuran in the reaction solution was distilled off under reduced pressure, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com