Synthesizing technology and device for improving nicosulfuron purity
A technology for the synthesis of nicosulfuron and nicosulfuron, which is applied in the field of synthesis technology and devices for improving the purity of nicosulfuron, can solve the problems of reducing the separation effect of the rectification tower, low yield content, long reaction time, etc., and achieve shortening Effect of reaction cycle, avoidance of environmental pollution, shortening of reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
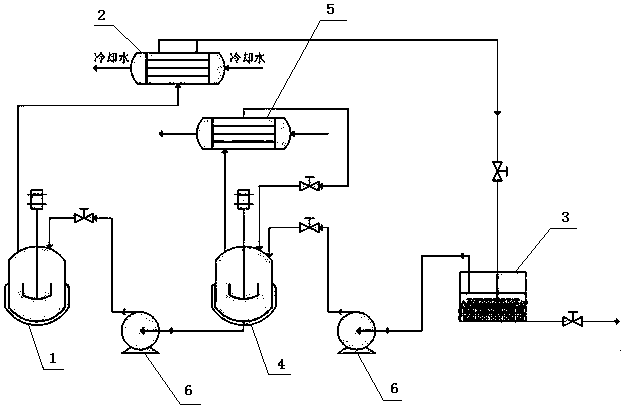
[0015] In the reactor, throw 120Kg (0.4Kmol) of urethane, 68.2Kg (0.44Kmol) of pyrimidinamine, and 900Kg of toluene. Throw 7.5kg (0.068Kmol) of calcium chloride in the calcium chloride storage tank, and throw 300Kg (3.26 Kmol) of toluene in the heating kettle. The reaction kettle and the heating kettle are heated at the same time, and the temperature of the reaction kettle is raised to boiling. The mixed steam of toluene and ethanol is condensed by the condenser and enters the calcium chloride storage tank to absorb ethanol. After that, the toluene enters the heating kettle to heat up and return to the reaction kettle. This operation is a continuous operation process. The content of ethanol in the toluene in the reaction kettle is lower than 0.0005% by sampling, the reaction is stopped, the temperature is lowered and centrifuged, and the product content after drying is 98.3%, and the yield is 98.4%.
Embodiment 2
[0017] In the reactor, throw 120Kg (0.4Kmol) of urethane, 68.2Kg (0.44Kmol) of pyrimidinamine, and 900Kg of toluene. Throw 7.9kg (0.072Kmol) of calcium chloride in the calcium chloride storage tank, and throw 300Kg (3.26 Kmol) of toluene in the heating kettle. The reaction kettle and the heating kettle are heated at the same time, and the temperature of the reaction kettle is raised to boiling. The mixed steam of toluene and ethanol is condensed by the condenser and enters the calcium chloride storage tank to absorb ethanol. After that, the toluene enters the heating kettle to heat up and return to the reaction kettle. This operation is a continuous operation process. The content of ethanol in the toluene in the reaction kettle is lower than 0.0005% by sampling, the reaction is stopped, the temperature is lowered and centrifuged, and the product content after drying is 98.4%, and the yield is 98.3%.
Embodiment 3
[0019] In the reactor, throw 120Kg (0.4Kmol) of urethane, 68.2Kg (0.44Kmol) of pyrimidinamine, and 900Kg of toluene. Throw 8.8Kg (0.08Kmol) of calcium chloride in the calcium chloride storage tank, and throw 300Kg (3.26 Kmol) of toluene in the heating kettle. The reaction kettle and the heating kettle are heated at the same time, and the temperature of the reaction kettle is raised to boiling. The mixed steam of toluene and ethanol is condensed by the condenser and enters the calcium chloride storage tank to absorb ethanol. After that, the toluene enters the heating kettle to heat up and return to the reaction kettle. This operation is a continuous operation process. The content of ethanol in the toluene in the reaction kettle is sampled to detect 0.0005%, the reaction is stopped, the temperature is lowered and centrifuged, and the product content after drying is 98.2%, and the yield is 98.3%.
[0020] In the process control of the above examples, compared with the existing co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com