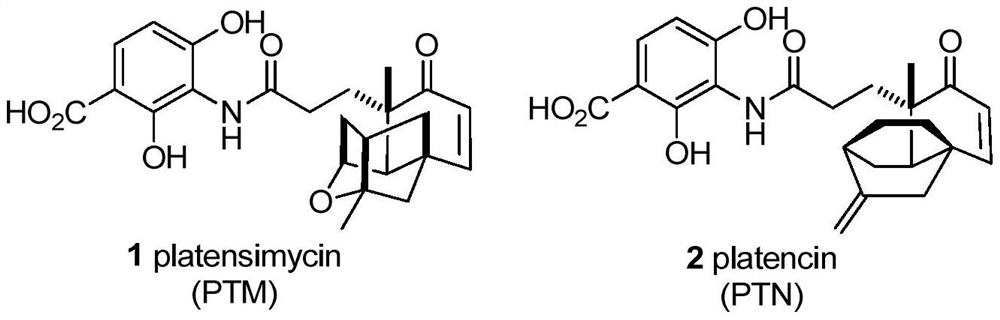
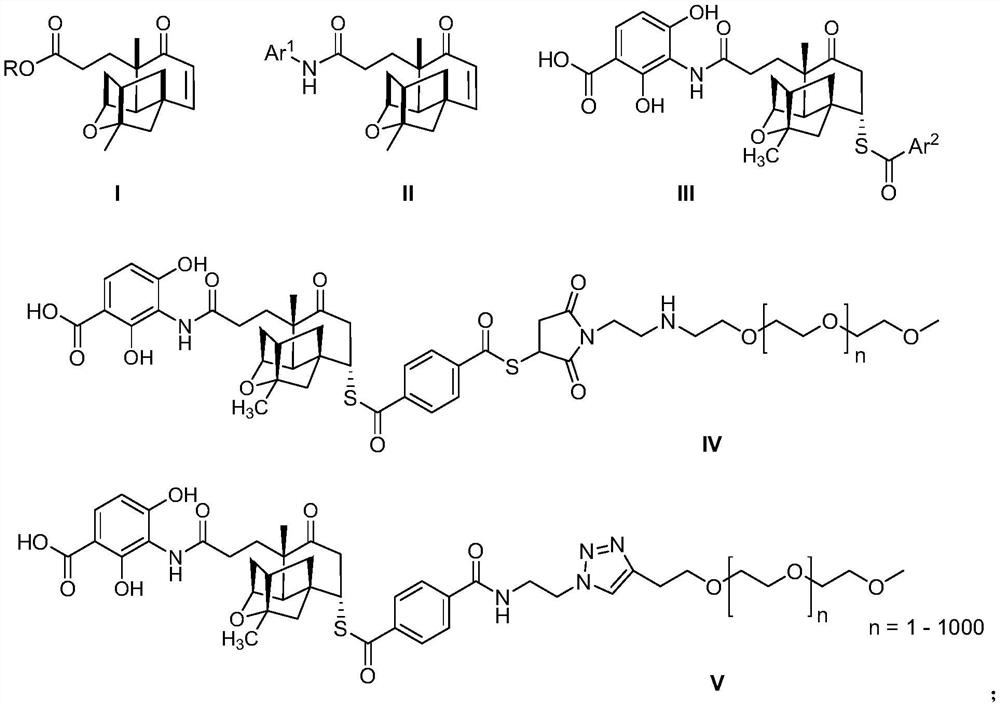
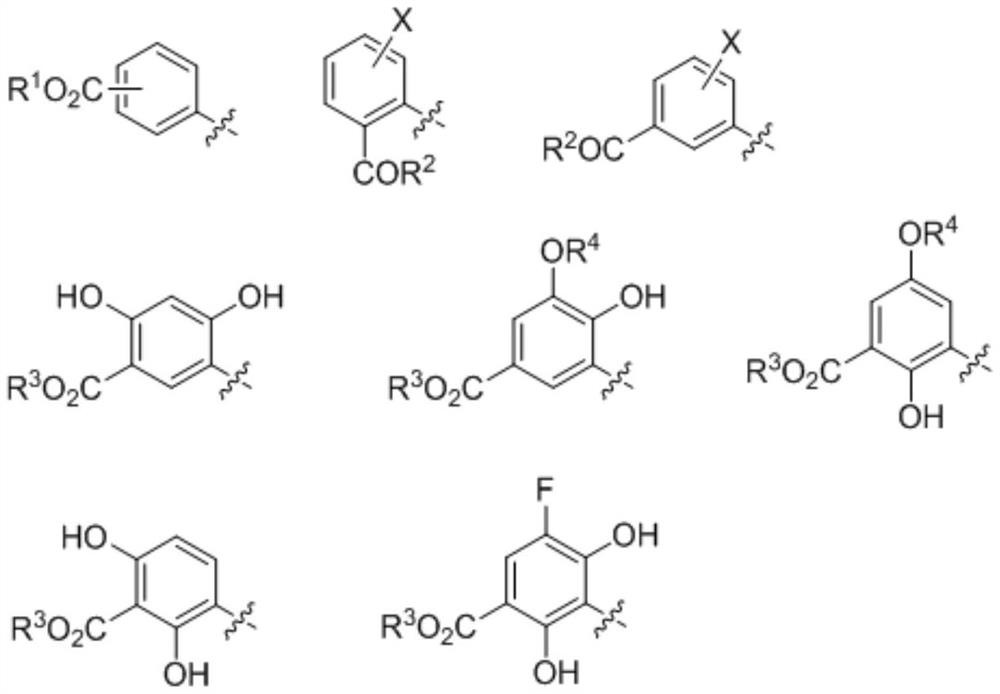
Palymycin analogue and its preparation method and application
A technology of platymycin and analogues, which is applied in antibacterial drugs, organic chemistry, etc., can solve the problems of not obtaining biological activity, etc., and achieve the effects of improving raw material utilization, simple operation, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1: Take platymycin (0.20 mmol), put it into a reaction bottle, add 7.0 ml of pure water, stir at room temperature for 5 minutes, and place it at -10°C. Weigh concentrated sulfuric acid (0.10mmol) and dissolve it in pure water, slowly add it dropwise to the reaction system, heat up to 80°C and stir for 12 hours, then remove the solvent by rotary evaporation to obtain a crude product; then pass through column chromatography (eluent: petroleum Ether: ethyl acetate = 1:20 ~ 1:5) was isolated to obtain platymycin acid PTMA-1 with a yield of 90%.
[0051] The structural formula of the product PTMA-1 is:
[0052] PTMA-1 is characterized by: 1 H NMR (500MHz, CDCl 3 )δ6.46(d, J=10.1Hz, 1H), 5.88(d, J=10.1Hz, 1H), 4.42(s, 1H), 2.41(t, J=6.5Hz, 1H), 2.38–2.31( m,2H),2.31–2.21(m,2H),2.13–2.05(m,1H),2.05–1.96(m,2H),1.87(dd,J=11.2,3.5Hz,1H),1.81–1.68( m, 2H), 1.60(d, J=11.2Hz, 1H), 1.44(s, 3H), 1.22(s, 3H);
[0053] 13 C NMR (101MHz, CDCl 3 )δ203.30, 177.62, 153.57, ...
Embodiment 2
[0055] Example 2: Weighed platymycin (0.20 mmol), put it into a reaction bottle, added 7.0 ml of methanol, stirred at room temperature for 5 minutes, and placed it at -10°C. Weigh concentrated sulfuric acid (0.10mmol) and dissolve it in methanol, slowly add it dropwise to the reaction system, heat up to 80°C and stir for 12 hours, then remove the solvent by rotary evaporation to obtain a crude product; then pass through column chromatography (eluent: petroleum ether : Ethyl acetate=1:20~1:5) separated to obtain platymycin acid PTMA-2 with a yield of 95%.
[0056] The structural formula of the product PTMA-2 is:
[0057] PTMA-2 is characterized by: 1 H NMR (500MHz, CDCl 3 )δ6.43(d, J=10.1Hz, 1H), 5.83(d, J=10.1Hz, 1H), 4.34(s, 1H), 3.60(s, 3H), 2.36(t, J=6.5Hz, 1H),2.32–2.13(m,5H),2.03(d,J=5.3Hz, 1H),2.00–1.91(m,3H),1.81(dd,J=11.1,3.4Hz,1H),1.70(ddd ,J=19.0,12.8,8.2Hz,3H), 1.57(d,J=11.1Hz,1H),1.39(s,3H),1.18(s,3H);
[0058] 13 C NMR (101MHz, CDCl 3 )δ203.21, 173.74, 15...
Embodiment 3
[0060] Example 3: Take platymycin (0.20 mmol), put it into a reaction bottle, add 7.0 ml of ethanol, stir at room temperature for 5 minutes, and place it at -10°C. Weigh concentrated sulfuric acid (0.10mmol) and dissolve it in ethanol, slowly add it dropwise to the reaction system, heat up to 80°C and stir for 12 hours, then remove the solvent by rotary evaporation to obtain a crude product; then pass through column chromatography (eluent: petroleum ether : Ethyl acetate=1:20~1:5) separated to obtain platymycin acid PTMA-3 with a yield of 95%.
[0061] The structural formula of the product PTMA-3 is:
[0062] PTMA-3 is characterized by: 1 H NMR (400MHz, CDCl 3 )δ6.43(d, J=10.1Hz, 1H), 5.84(d, J=10.1Hz, 1H), 4.35(s, 1H), 4.10–4.05(m, 2H), 2.37(t, J=6.5 Hz,1H),2.32(s,1H),2.30–2.11(m,3H), 2.03(dd,J=6.3,5.3Hz,1H),1.99–1.94(m,2H),1.81(dd,J= 11.1,3.6Hz,1H),1.77–1.64(m,2H),1.61–1.54(m,1H),1.40(s,3H),1.19(s,3H),1.19(dd,J=6.3,3.2Hz ,3H);
[0063] 13 C NMR (101MHz, CDCl 3 )δ20...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com