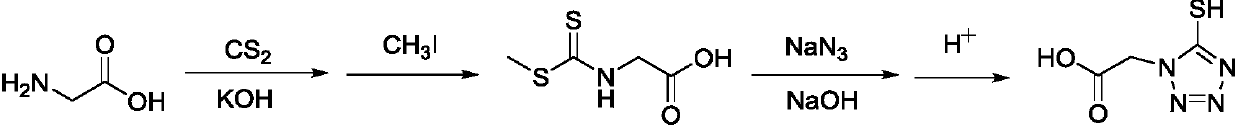
5-tetrazole-thione acetic acid and preparation method of sodium salt thereof
A technology of mercaptotetrazolium acetic acid disodium salt and mercaptotetrazolium acetic acid is applied in the field of preparation of 5-mercaptotetrazolium acetic acid and its sodium salt, and can solve the problems of toxicity, high raw material price, low yield and the like, To achieve the effect of reducing process costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
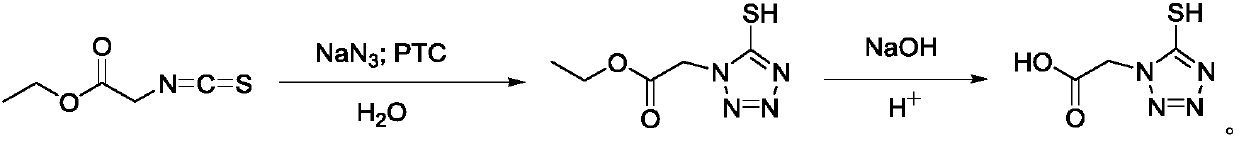
[0015] Embodiment 1: the preparation of 5-mercaptotetrazolium acetic acid
[0016] Add (71.5g, 1.1mol) sodium azide, (18.5g, 0.1mol) benzyltriethylammonium chloride, and 150mL water into a 500mL three-necked flask. In a nitrogen atmosphere, the temperature of the system was raised to 60° C. and kept stirring. (145.0 g, 1.0 mol) ethyl isothiocyanoacetate was slowly added dropwise to the system. After the drop was completed, the system was heated to 75° C. for 4 h. After the reaction, the temperature of the system was lowered to 5° C., and the pH of the system was adjusted to about 12 with 50% NaOH solution, and stirred for 0.5 h until the pH was stable. The temperature of the system was raised to 75°C, and the reaction was kept for 5 hours. After the reaction, the system was cooled to room temperature, and hydrochloric acid (150.0 g, wt%=31%) was slowly added to the system. After the addition, react at room temperature for 2h. The reaction system was extracted with (200 mL×...
Embodiment 2
[0018] Embodiment 2: Preparation of monosodium salt of 5-mercaptotetrazolium acetic acid
[0019] Add (100.0 g, 0.63 mol) 5-mercaptotetrazolium acetic acid and 150 mL of water into a 500 mL three-neck flask. The system was stirred at room temperature, and NaOH solution (250.0 g, wt% = 10%) was slowly added to the system, and the temperature of the system was raised to 90° C. for 2 hours after the addition. After the reaction was completed, the system was lowered to 10°C, filtered, and the filter cake was vacuum-dried at 60°C to obtain the product (104.0 g, 0.57 mol) of 5-mercaptotetrazolium acetic acid monosodium salt, with a yield of 90.0%.
Embodiment 3
[0020] Embodiment 3: Preparation of 5-mercaptotetrazolium acetic acid disodium salt
[0021] Add (100.0 g, 0.63 mol) 5-mercaptotetrazolium acetic acid and 150 mL of water into a 500 mL three-neck flask. The system was stirred at room temperature, and NaOH solution (500.0 g, wt% = 10%) was slowly added to the system, and the temperature of the system was raised to 90° C. for 2 hours after the addition. After the reaction was completed, the system was lowered to 10°C, filtered, and the filter cake was vacuum-dried at 60°C to obtain the product (117.0 g, 0.57 mol) of 5-mercaptotetrazolium acetic acid disodium salt with a yield of 90.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com