Method for obtaining homozygous mutation of seamless modification
A homozygous, seamless technology, applied in the field of molecular biology, can solve the problems of low efficiency of precise and seamless gene modification, troublesome construction of plasmids, lack of screening system, etc., to save manpower, material resources and time, facilitate disease models, and select the range wide range of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 CRISPR / Cas9 combined with piggyBac transposon carrying puro / TK resistance screening to efficiently obtain heterozygous mutations
[0034] experimental method
[0035] 1. Cell culture and transfection
[0036] Seed iPS cells on cell culture plates pre-coated with Matrigel (BD Biosciences), and use mTeSR TM 1 (Stemcell Technologies) at 37°C, 5% CO 2 cultured in a cell culture incubator. Before Lipofectamine transfection, iPS cells were divided into 4×10 per well 5 ~5×10 5 The density was passaged on six-well plates coated with Matrigel, and mTeSR containing 10 μM ROCK inhibitor (Sigma) TM 1 was incubated overnight. On the second day after passaging, iPS cells were co-transfected with 6 μg pCas9_NeoR, 3 μg sgRNAplasmid and 50 nM ssODN (90 bpssODN), and the cells in the other well were transfected with 2 μg pmaxGFP alone as a control for transfection efficiency. The transfection process was strictly in accordance with Lipofectamine TM 3000's official opera...
Embodiment 2
[0056] Example 2 CRISPR / Cas9 combines piggyBac transposons containing puro / TK and hygR / TK resistance screening respectively to obtain homozygous mutations efficiently
[0057] experimental method
[0058] The experimental method is the same as that used in Example 1.
[0059] 1. Cell culture and transfection
[0060] With embodiment 1.
[0061] 2. iPS single cell clone culture
[0062] With embodiment 1.
[0063] 3. Construction of Cas9 and PB-based plasmids
[0064] With embodiment 1.
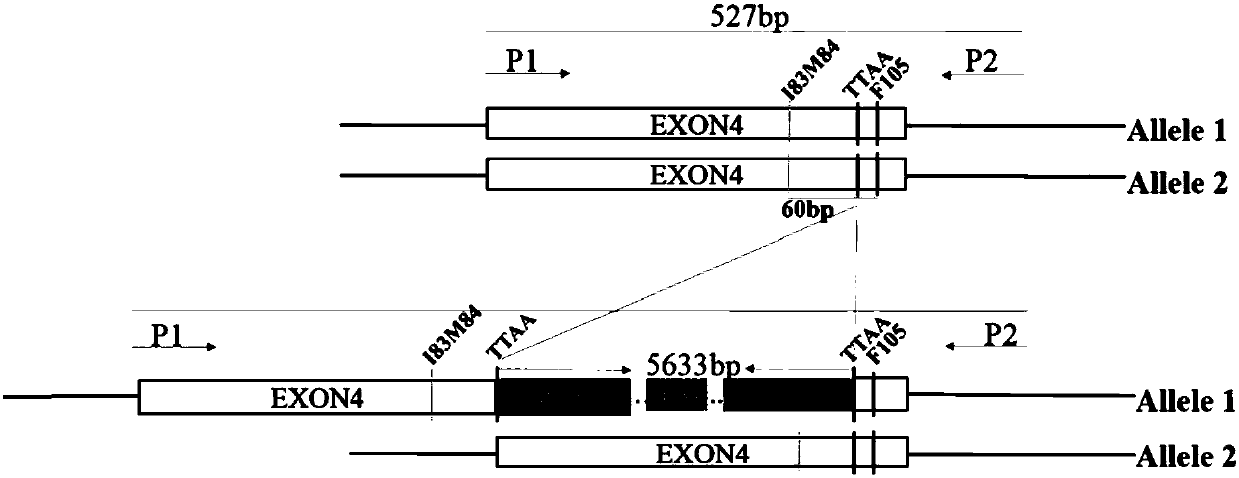
[0065] 4. Obtain homozygous mutation
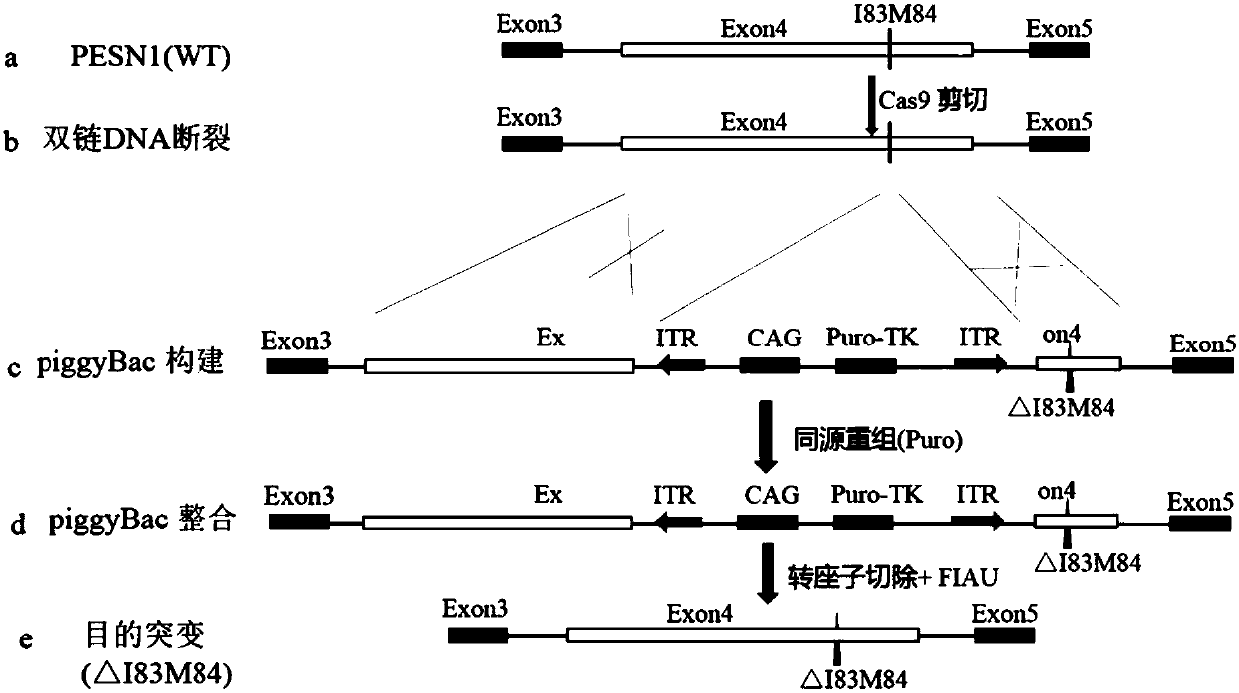
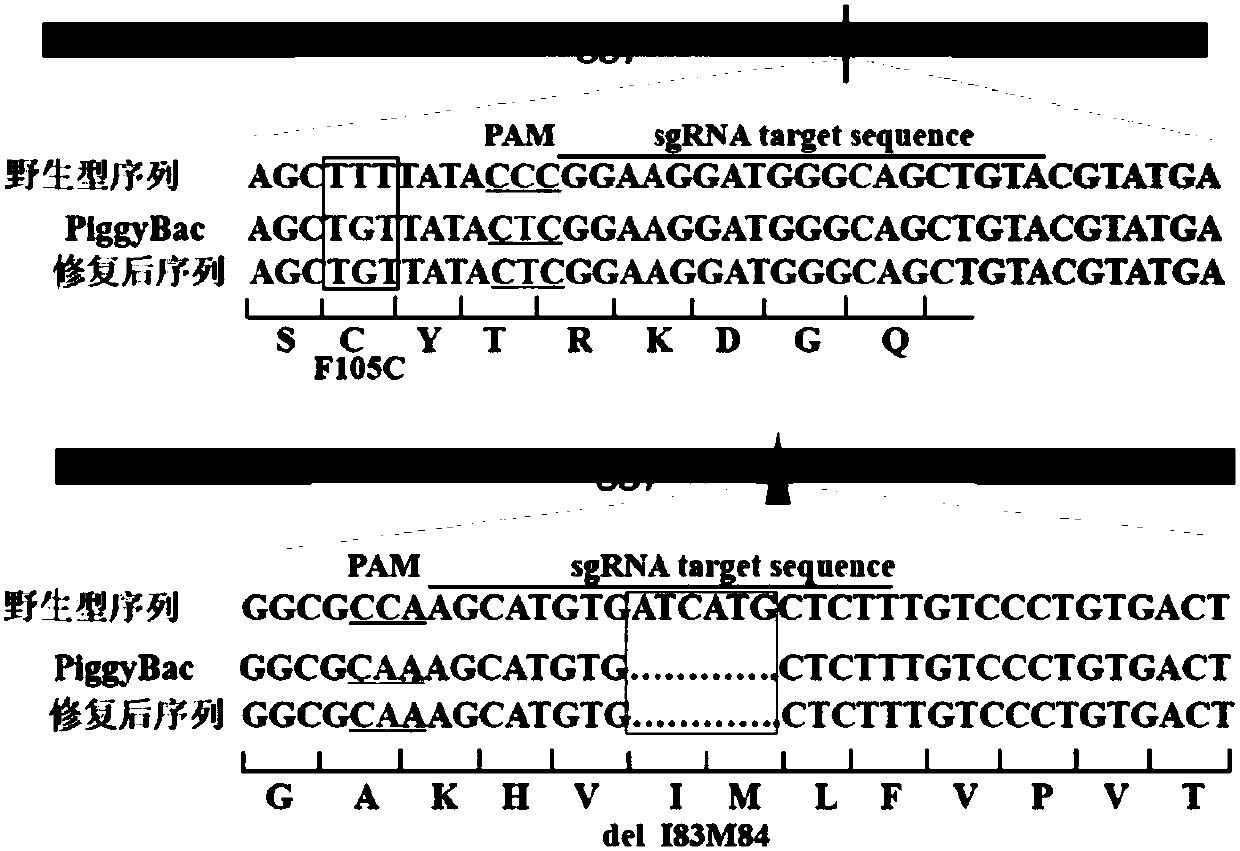
[0066] The following still takes PSEN1ΔI83M84 as an example to illustrate the process of using CRISPR / Cas9 and the new piggyBac transposon system to obtain seamless homozygous mutations, such as Figure 2A Shown:
[0067] (a) First, determine that the site to be mutated is on exon 4 of PSEN1;
[0068] (b) using Cas9 to cut at the target site to generate DSB;
[0069] (c) Construction of piggyBac transposons (SEQ ID No: 7 and SEQ ID No: 8) carryi...
Embodiment 3
[0076] Example 3 Cells carrying AD-related gene mutations acquire gene mutation-dependent phenotypic changes experimental methods
[0077] 1. Differentiation of cortical neurons
[0078] Human iPS cells are differentiated toward neural stem cells, and the cultured iPS cells are scraped into small clonal clusters with a cell scraper at 2.5-3.0×10 4 cells / cm 2 The density of PSC Neural Induction Medium (Life Technologies) plus Y23632 (sigma) was used to culture on a 6-well plate pre-coated with matrigel (BD Biosciences), the medium was changed every two days, and the culture was continued for 7 days. After 7 days, single cells were digested with Accutase (Sigma) at 1.0-1.2×10 5 cells / cm 2 density for expansion.
[0079] For cortical neuron differentiation, neural stem cells were plated at 2 × 10 5 cells / cm 2 The density of N2B27 medium (DMEM-F12: Neural Basal medium 1:1, 2% B27, 1% N2, 1% non-essential amino acids (non-essential aminoacids), 2mM Glutamax, 100U penicillin p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com