Breeding method for establishing primary biliary cholangitis model mouse through bile duct antigen immunity
A model mouse, antigen immunization technology, applied in the field of medical biology, can solve problems such as serious disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1. The process of establishing model mice by immunization with bile duct antigens.
[0053] During this process, all mice were wild-type C57BL / 6 background mice purchased from Shanghai Slack Experimental Animal Co., Ltd., and raised in the Specific Pathogen Free (SPF) laboratory animal center of the School of Life Sciences, University of Science and Technology of China. Environment.
[0054] We used collagenase IV (purchased from Sigma-Aldrich, the working concentration of collagenase IV is 0.075% by mass / volume ratio) dissolved in DMEM high-glucose medium (purchased from Thermo Fisher Scientific) to perfuse the mouse portal vein, and the perfusion The temperature was controlled at a constant temperature of 37°C, and after continuous perfusion for 10 minutes (perfusion rate was 5ml / min), the hepatic parenchymal cells attached to the periphery of the bile duct were gently brushed off with a soft brush to obtain a complete bile duct of the mouse. Cut the obtained...
Embodiment 2
[0055] Example 2. Isolation of mononuclear cells in various organs
[0056] Isolation of mononuclear cells from the liver: the liver of the mouse was removed, ground with PBS containing 2% BSA, filtered through a steel mesh, centrifuged at 1500 rpm for 5 min, and the precipitate was taken. The cell pellet was subjected to density gradient centrifugation with 40% percoll (GE Healthcare) for 20 min, and the cell pellet was collected. The erythrocytes in the pellet were lysed for 10 min with the erythrocyte lysate, and the cells were counted under a microscope by a hemocytometer.
[0057] Separation of spleen mononuclear cells: remove the mouse spleen, grind it with two glass slides and PBS containing 2% BSA, filter through nylon mesh, centrifuge at 1500rpm for 5min, and then add red blood cell lysate (purchased from Beyond Biotechnology Co., Ltd. company) lysed the erythrocytes in the cell pellet for 10 min, and then counted the cells under a microscope with a hemocytometer.
...
Embodiment 3
[0060] Example 3. Histopathological examination
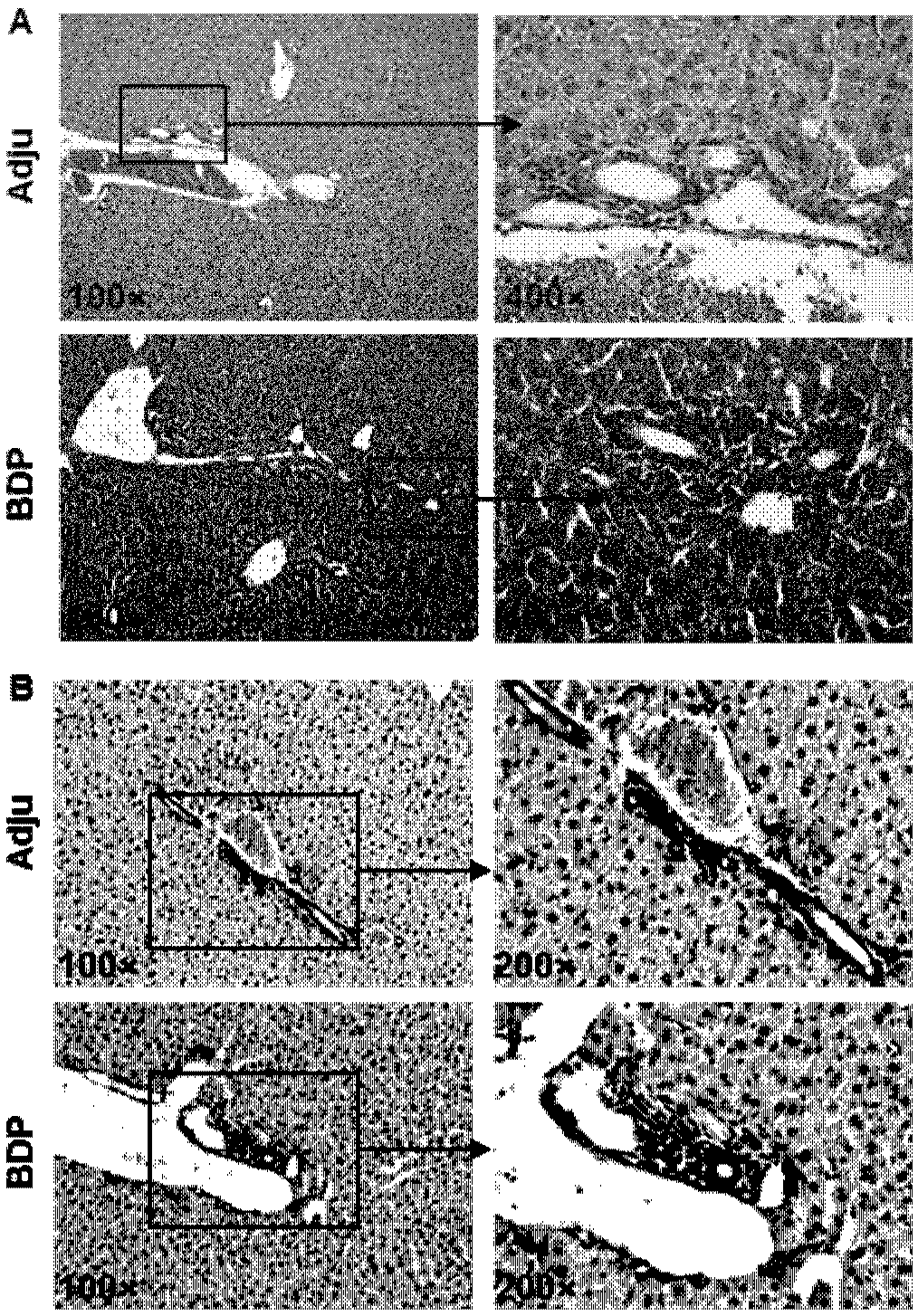
[0061] The mouse liver tissues were fixed in 4% neutral formaldehyde for 1-2 days, dehydrated and embedded in paraffin. Paraffin-embedded liver tissue was sectioned into 4-μm slices, and all slices were deparaffinized and stained with H&E to show infiltration of the portal area in the liver, or deparaffinized and immunohistochemically stained with CK-19 to show inflammation around the bile ducts infiltration.
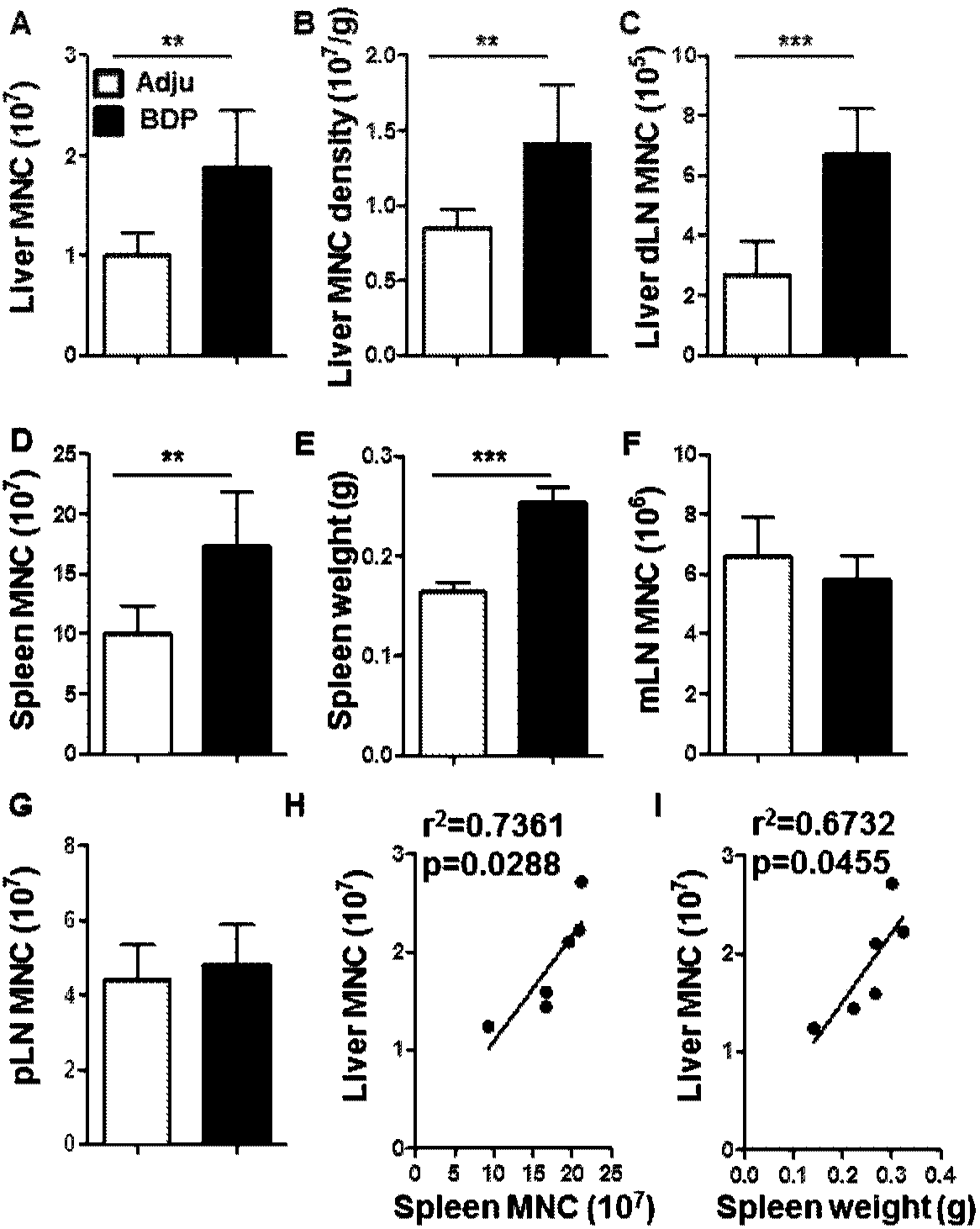
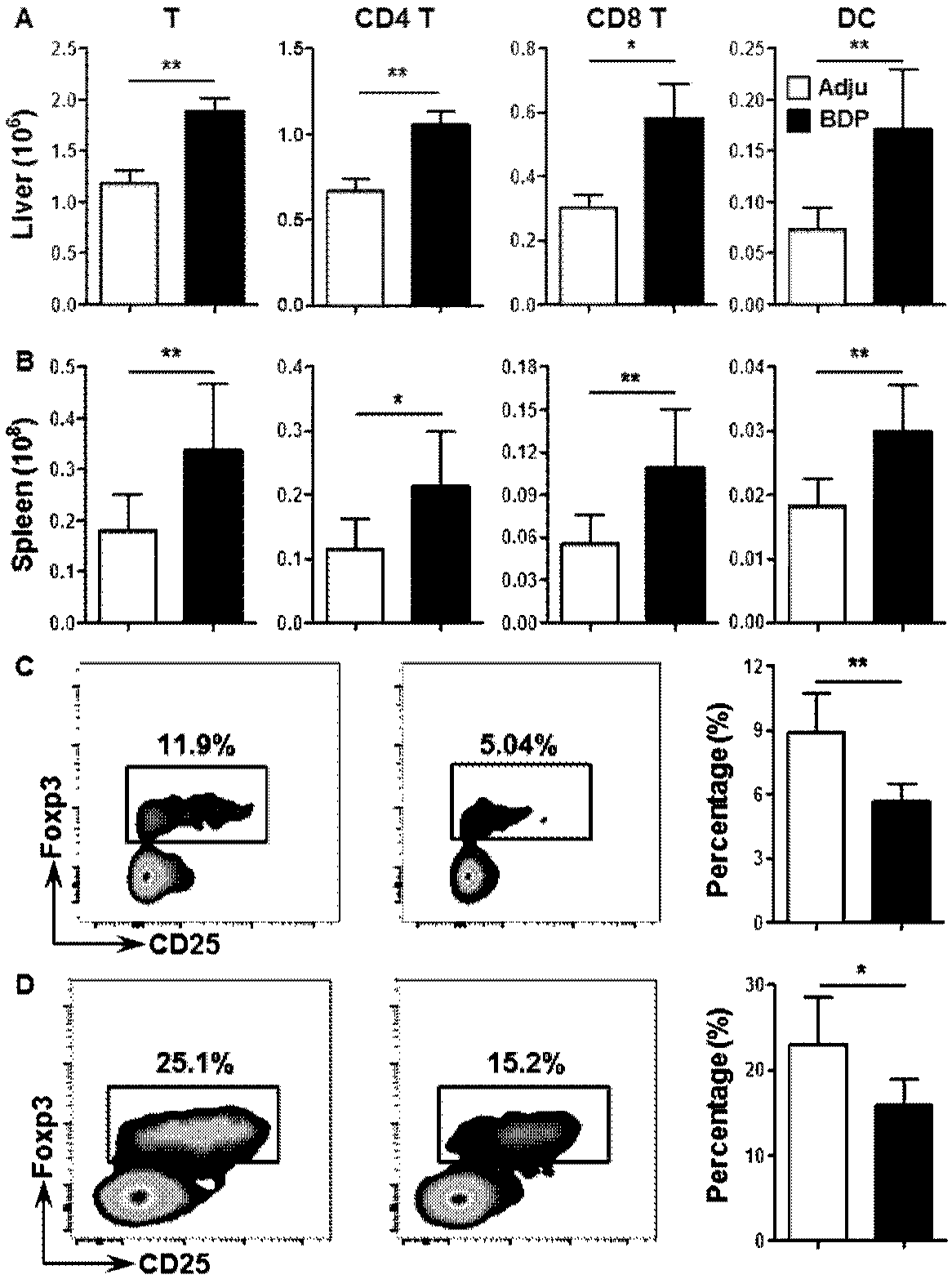
[0062] see results figure 2 HE staining and CK-19 immunohistochemical staining showed that the portal area in the liver of the model mice had obvious inflammatory infiltration, and the specific location of the inflammatory infiltration was around the bile duct. Patients with primary biliary cholangitis usually present with lymphocytic infiltration and bile duct damage in the intrahepatic portal bile duct area, figure 2 The results of the histopathology verified that the performance of the model mice was consistent w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com