Cyclodextrin-metal organic framework (CD-MOF) composite microsphere and preparation method thereof
A metal-organic framework and composite microsphere technology, applied in non-active ingredients medical preparations, microcapsules, pharmaceutical formulations, etc., can solve the problems of drug burst release, structural collapse, easy collapse of CD-MOF, etc., to achieve good protection effect of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
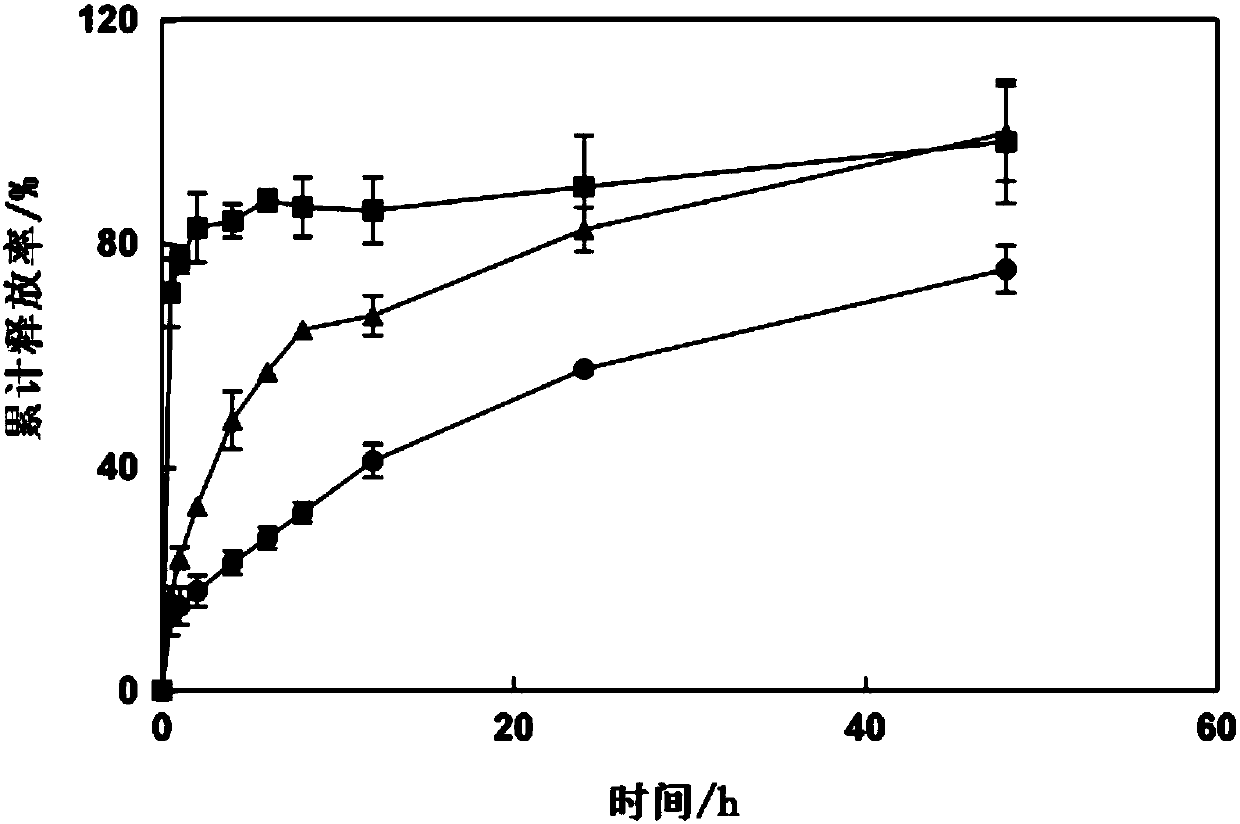
[0232] (c) The preparation method of the present invention can maintain the structural integrity of CD-MOF.
[0233] (d) The preparation method of the present invention has simple and controllable process, low cost and good effect.
Embodiment 1
[0239] The prescription is shown in the table below:
[0240]
[0241] The preparation process is as follows:
[0242] Weigh 50 mg of ibuprofen-loaded CD-MOF and disperse it in 3 mL of acetone, ultrasonically disperse it evenly, weigh 450 mg of polyacrylic acid resin (MOF: polyacrylic acid resin ratio 1:9, w / w) and dissolve it, then add 120 mg Aluminum stearate (dispersant) is dispersed evenly by ultrasonication for 5 minutes, which is the dispersed phase. Add the dispersed phase containing aluminum stearate to liquid paraffin (ice-bathed to 10°C), place the above suspension in an ice-water bath at 10°C, stir magnetically (500rpm, 30s), and disperse with a disperser (10000rpm, 5min) to obtain an S / O / O type emulsion. Place the above-mentioned emulsion on a magnetic stirrer, and under the condition of 500rpm stirring, slowly raise the temperature from 10°C to 35°C (the setting of magnetic stirring heating is 65, and the heating rate is 10°C·min-1), and then continue to stir...
Embodiment 2
[0245] The prescription is shown in the table below:
[0246]
[0247] The preparation process is as follows:
[0248] Weigh 50 mg of lansoprazole-loaded CD-MOF and disperse it in 3 mL of acetone, ultrasonically disperse it evenly, weigh 450 mg of polyacrylic acid resin (MOF: polyacrylic acid resin is 1:9, w / w) and dissolve in it, then add 120mg of aluminum stearate (dispersant) was ultrasonically dispersed for 5 minutes to form a dispersed phase. Add the dispersed phase containing aluminum stearate to liquid paraffin (ice-bathed to 10°C), place the above suspension in an ice-water bath at 10°C, stir magnetically (500rpm, 30s), and disperse with a disperser (10000rpm, 5min) to obtain an S / O / O type emulsion. Place the above-mentioned emulsion on a magnetic stirrer, and under the condition of 500rpm stirring, slowly raise the temperature from 10°C to 35°C (the setting of magnetic stirring heating is 65, and the heating rate is 10°C·min-1), and then continue to stir for 3h (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com