Application of EVI-1 inhibitor for preparing medicines for treating vascular anomaly remodeling diseases
A vascular abnormality, EVI-1 technology, applied in the field of biomedicine, can solve the problem of not inhibiting EVI-1 against abnormal vascular remodeling, and achieve the effect of reducing abnormal intimal neogenesis, reducing toxic side effects, and promoting specific gene expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
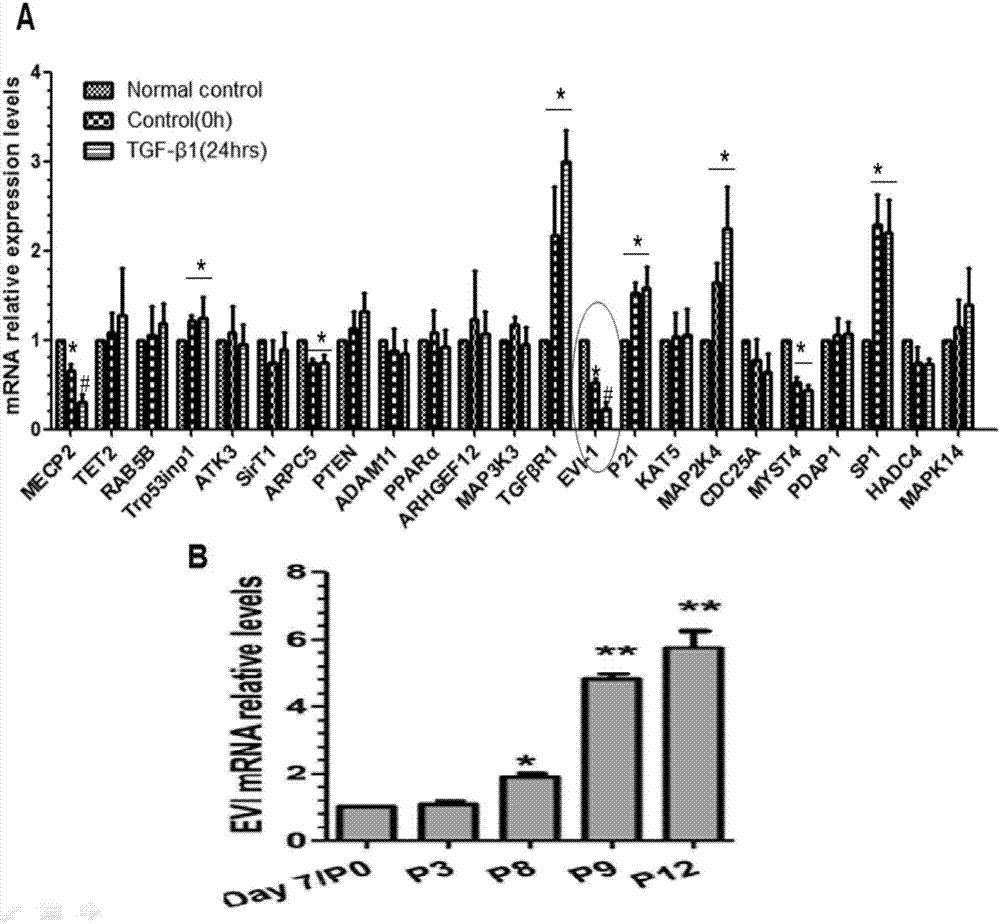
[0026] Example 1 EVI-1 is involved in the process of smooth muscle cell phenotype transformation.
[0027] Primary mouse aortic smooth muscle cells were isolated and cultured by enzymatic digestion. The vascular smooth muscle cells used in the experiment were selected from P3-P12. Primary aortic smooth muscle cells were cultured in 10% FBS-DMEM medium (FBS was purchased from Gibco, and DMEM was purchased from Hyclone) in a 5% CO2 incubator at 37°C.
[0028] Vascular smooth muscle cell stimulation group: normal culture for 48 hours, serum-free starvation for 48 hours, and serum-free starvation for 24 hours + 5ng / ml TGF-β stimulation for 24 hours. After smooth muscle cells were treated according to the above grouping, cellular RNA was collected, and RT-qPCR was used to detect the expression changes of related target genes during the process of smooth muscle cell phenotype transformation. figure 1 The results of A showed that EVI-1 decreased significantly after 24 hours of seru...
Embodiment 2
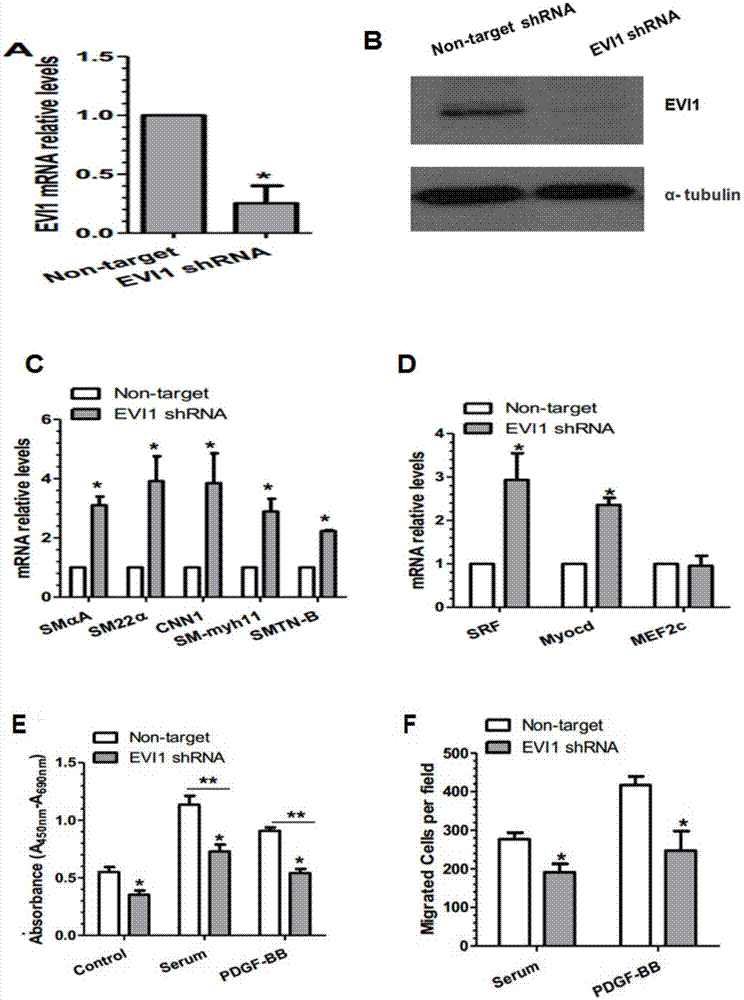
[0031] Example 2 EVI-1 regulates phenotypic transformation of smooth muscle cells.
[0032] Establishment of EVI-1 gene stable knockdown cell lines. EVI-1 shRNA plasmid construction, shRNA sequence: Evi1-shRNA-F: 5'-CCGGCAGGTACTGTGGCAAGATATTCTCGAGAATATCTTGCCACAGTACCTGTTTTTG-3' (SEQ ID NO.2); Evi1-shRNA-R: 5'-AATTCAAAAACAGGTACTGTGGCAAGATATTCTCGAGAATATCTTGCCACAGTACCTG-3' (SEQ ID NO.3). After the construction of the plasmid is completed, further virus packaging is required to play a role: (1) 293T cells are routinely cultured and passaged, and the passage ratio is optimal to make the cell density reach 80% on the second day according to the experimental requirements. (2) Use TransIT-X2 (mirusbio company reagent to co-transfect Evi1-shRNA plasmid or control plasmid, pUMVC and pCMV-VSV-G into 293T cells (strictly follow the company's instructions). (3) Change to conventional culture after overnight transfection (4) After 48 hours, the cell supernatant was collected, centrifuged, f...
Embodiment 3
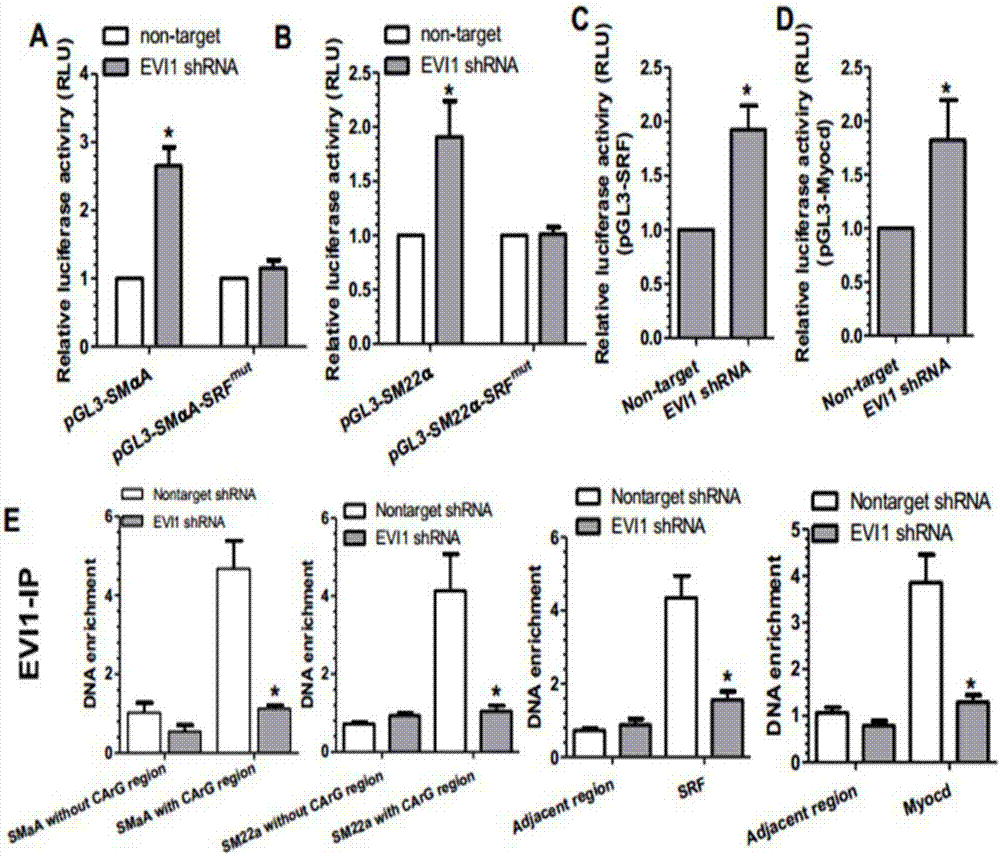
[0038] Example 3. EVI-1 transcriptional repression gene list of vascular smooth muscle cell-specific contraction.
[0039]Cell transfection: Use TransIT-X2TM transfection reagent to transfect relevant plasmids into vascular smooth muscle cells (one well in a 24-well culture plate). (1) The smooth muscle cells were routinely cultured and subcultured, and the subculture ratio was optimal to make the cell density reach 80% on the second day according to the experimental requirements. (2) Mix TransIT-X2TM Transfection Reagent before use. (3) Prepare the following reagents according to the protocol. Serum-free DMEM 50ul; plasmid 200ng; TransIT-X2 1.5ul (4) Stand at room temperature for 30 minutes or more to allow the mixed reagents to fully combine (5) Replace the cell medium and add freshly prepared 2% FBS+DMEM medium. (6) Evenly drop the transfection mixture into the well plate. (7) Collect cell samples for testing after 48 hours.
[0040] Luciferase detection: Use the Dual-L...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com