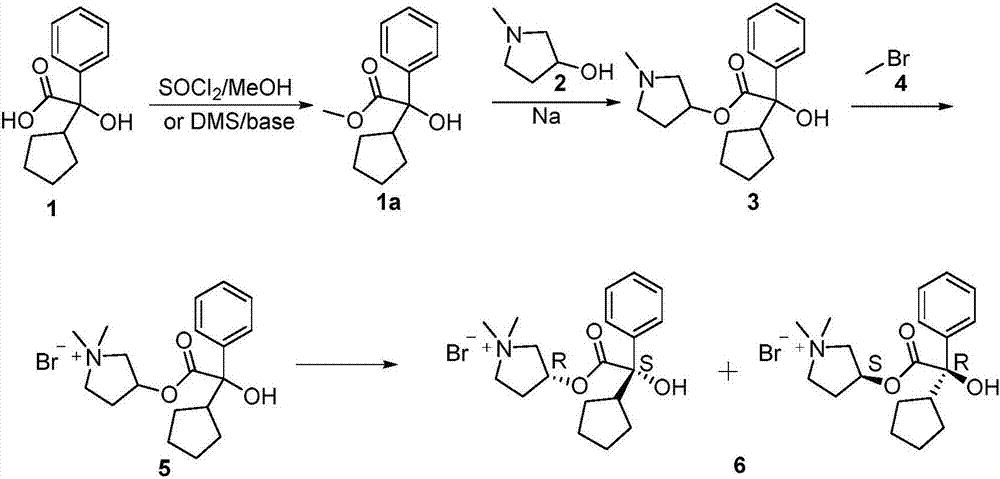
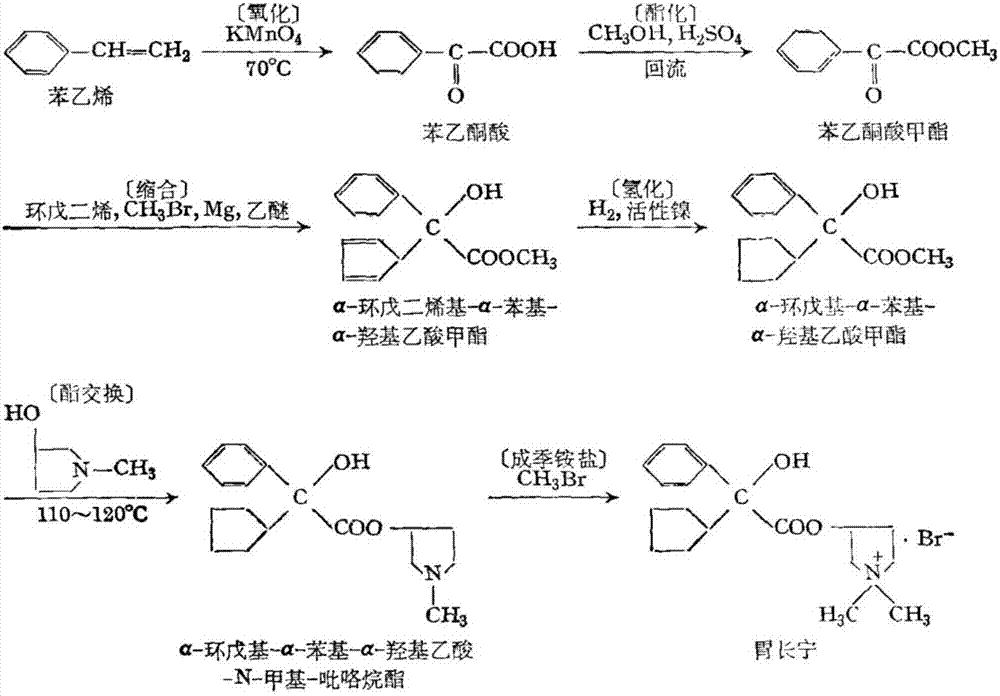
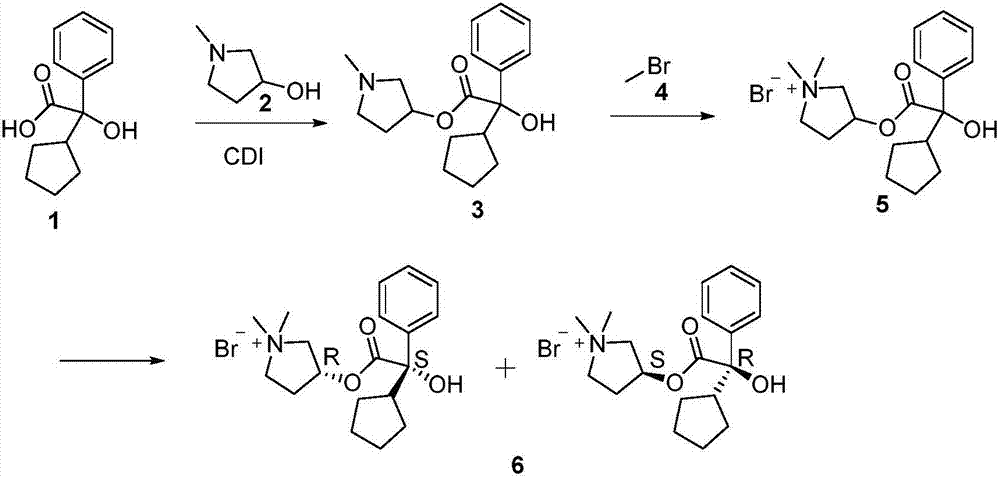
Preparation method of glycopyrronium bromide
A technology of glycopyrronium bromide and intermediate, applied in the preparation field of glycopyrronium bromide, can solve problems such as unfavorable industrialized production, high production operation requirements, low intermediate yield, etc., and achieves low production cost, simplified aftertreatment, The effect of increasing productivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] The preparation method of glycopyrrolate of the present invention includes the following steps:
[0034] 1. Add α-cyclopentylmandelic acid to NaOH DMF solvent, react at 30-80℃ for 0.5-2h, then add a little excess benzyl chloride to the reaction solution, and then heat up to 90-130℃ React for 1-72h. Water is continuously separated during the reaction. After the reaction is completed, purified water is added to the reaction solution, and the small polar organic solvent is extracted and separated. The pH of the aqueous phase is adjusted to 5-6 to precipitate solids, and intermediate I is obtained by filtration. -Benzyl-protected mandelic acid;
[0035] 2. Add the intermediate I obtained in step 1 to a slight excess of 1-methyl-3-pyrrolidinol in THF solution, slowly add a catalytic amount of concentrated sulfuric acid dropwise at 40-75°C, and cool down after the reaction is complete To room temperature, add lye dropwise to adjust pH=12-13, and extract with organic solvent to ob...
Embodiment 1
[0042] The preparation method of glycopyrrolate in this embodiment is as follows:
[0043] 1. Add 220g of α-cyclopentylmandelic acid to 600g of DMF solvent containing 88g of NaOH, stir and react at 60℃ for 1h, then add 139g of benzyl chloride dropwise to the reaction solution, after dripping, heat to 110℃ and stir for 3h. Water was continuously separated during the process. After the reaction was completed, 1kg of purified water was added to the reaction solution, and then 100g of ether was added for liquid separation. The aqueous phase was adjusted to pH 5-6 with dilute hydrochloric acid (4M) to precipitate solids, and intermediate I was obtained by filtration. -Benzyl-protected mandelic acid, 285g;
[0044] 2. Add 285g of Intermediate I obtained in step 1 to 400g of THF solution containing 111g of 1-methyl-3-pyrrolidol, and slowly add 19.6g of concentrated sulfuric acid (98wt%) at 70°C. After the reaction is complete , Cooled to room temperature, 30wt% NaOH solution was added dr...
Embodiment 2
[0049] The preparation method of glycopyrrolate in this embodiment is as follows:
[0050] 1. Add 440g of α-cyclopentylmandelic acid to 1200g of DMF solvent containing 176g of NaOH, stir and react at 60°C for 1.5h, then add 278g of benzyl chloride dropwise to the reaction solution, and then heat up to 110°C and stir for 4h. Water was continuously separated during the reaction. After the reaction was completed, 2kg of purified water was added to the reaction solution, and then 200g of ether was added for liquid separation. The aqueous phase was adjusted to pH 5-6 with dilute hydrochloric acid (4M) to precipitate solids, and intermediate I was obtained by filtration. ——Mandelic acid protected by benzyl group, 562g;
[0051] 2. Add 562g of Intermediate I obtained in step 1 to 700g of THF solution containing 222g of 1-methyl-3-pyrrolidinol, and slowly drop 35g of concentrated sulfuric acid (98wt%) at 70°C. After the reaction is complete, Cool to room temperature, add 30wt% NaOH soluti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com