Hagfish oral gland cystatin, preparation method and application
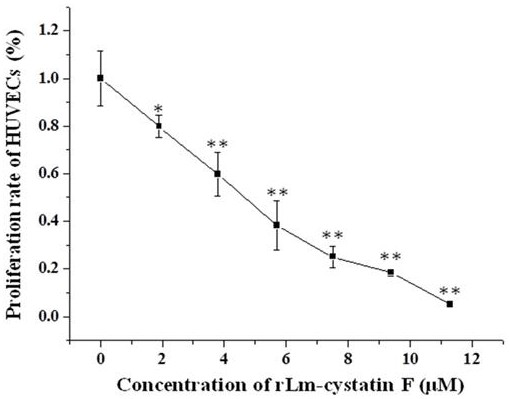
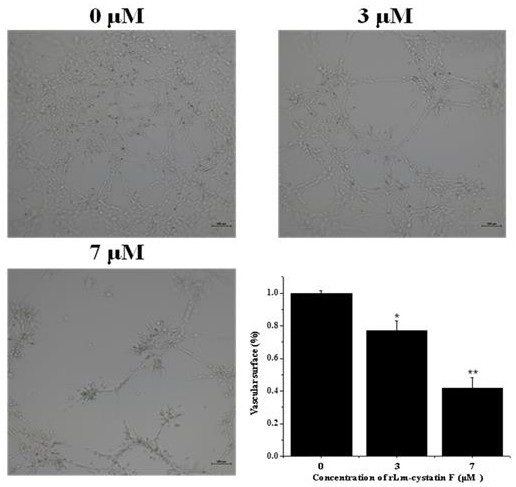
A technology of oral gland and hagfish, which is applied in the field of hagfish oral gland CystatinF, preparation and application, and can solve the problems such as the molecular characteristics and biological functions of hagfish oral gland cystatinF, the small amount of expression, and the complicated operation. , to achieve the effect of inhibiting the differentiation of vascular endothelial cells, short expression cycle and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
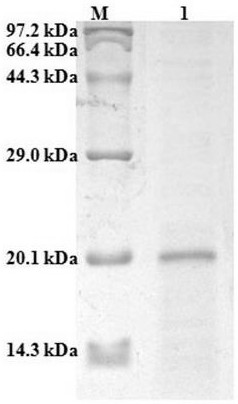
[0028] The present invention identified cystatin F ( Lampetra morii cystatin F, Lm-cystatin F), the open reading frame sequence of Lm-cystatin F contains 459 nucleotides and consists of 152 amino acids. The DNA sequence and amino acid sequence are shown in SEQ ID NO: 1, where M1-A21 is signal peptide. Lm-cystatin F has an apparent molecular weight of 17.1 kDa and a theoretical isoelectric point of 10.31. It contains two pairs of disulfide bonds and has the typical structural domains of this family of proteins: glycine G at the N-terminal of the sequence, and Q at the middle of the sequence. X V X G motif and proline and tryptophan (PW) at the C-terminus of the sequence. Sequence analysis showed that cystatin F from northeast hagfish had low homology with cystatins from other species.
[0029] The preparation method is carried out according to the following steps:
[0030] a. the DNA sequence is cloned into the expression vector pCold I as the gene shown in SEQ ID NO:2 an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com