Expression and application of thymosin-repeated protein Cq-TRP1 resisting WSSV (White Spot Syndrome Virus) infection in pichia pastoris
A technology of thymosin and protein, applied in the direction of thymopoietin, application, animal/human protein, etc., can solve problems such as obstacles to prevention and control work
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
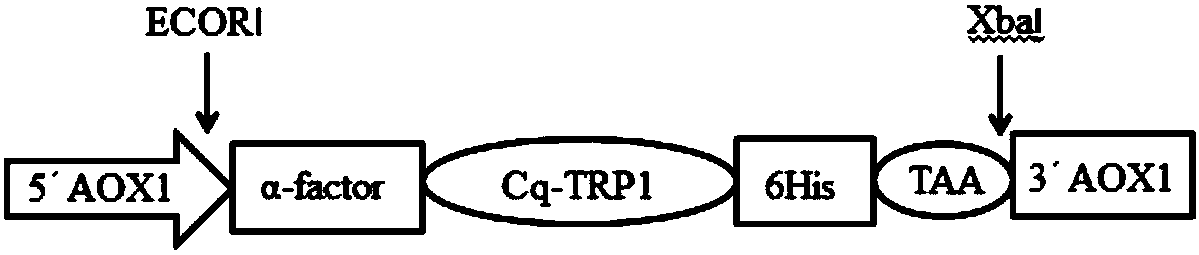
[0049] The construction of embodiment 1 red claw crayfish thymosin Cq-TRP1 eukaryotic expression vector
[0050] According to the multiple cloning site of the pPICZaA vector, a specific upstream primer F1 and a downstream primer R1 for amplifying the ORF of the Cq-TRP1 (cDNA) gene encoding the red-clawed crayfish were designed. An EcoR I restriction site was added to the 5' end of the upstream primer F1; an XbaI restriction site, a stop codon and a base encoding His-tag were added to the 5' end of the downstream primer R1.
[0051] Upstream primer F: 5'-CCGGAATTCAGCACCGAATCCTCACTCA-3', downstream primer R: 5'-GCTCTAGATTAATGATGATGATGATGGTGGGCTTTCTTCTCCTGCTCAATCT-3'.
[0052] The PCR reaction conditions were: pre-denaturation at 94°C for 3min; denaturation at 94°C for 30s; annealing at 60°C for 30s; extension at 72°C for 30s; repeat 30 cycles; extension at 72°C for 10min.
[0053] The PCR product was recovered using an agarose gel purification kit, and the recovered PCR product...
Embodiment 2
[0055] Example 2 Induced expression of pPICZaA-Cq-TRP1 recombinant plasmid in Pichia pastoris GS115
[0056] The correctly sequenced pPICZaA-Cq-TRP1 plasmid was linearized by BamHI digestion, transformed into Pichia pastoris GS115 competent cells by electric shock method, and the expression was induced by methanol.
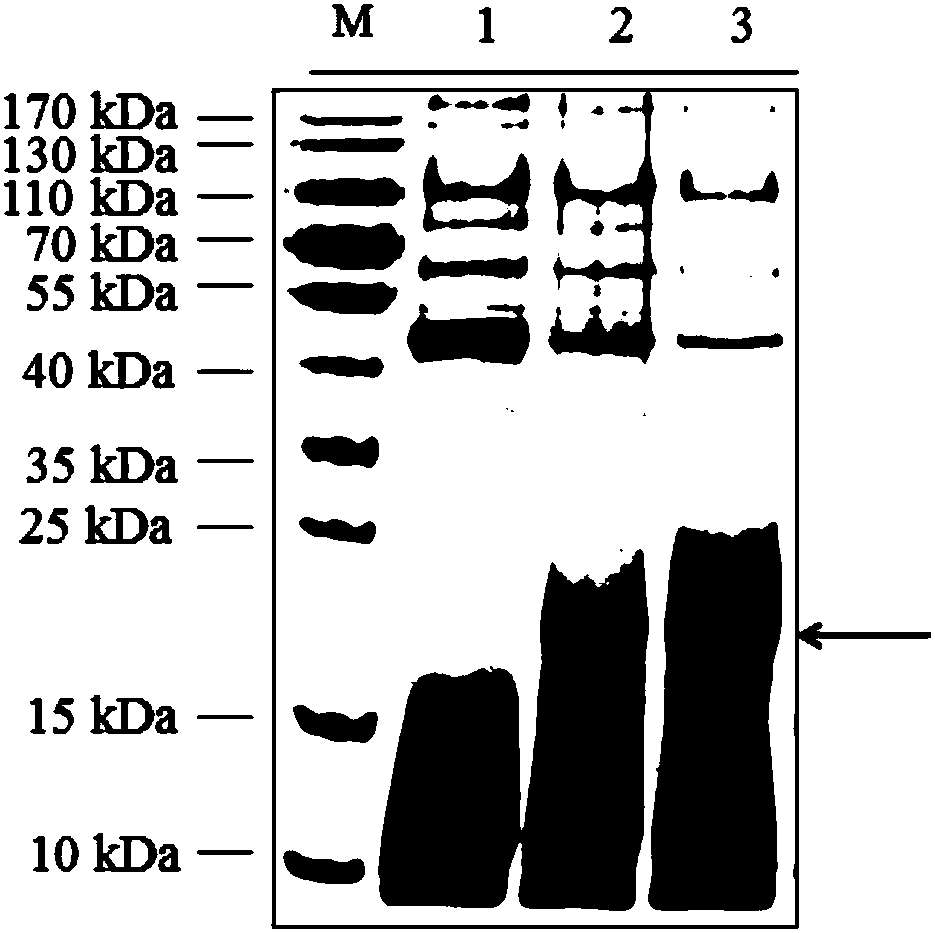
[0057] The results showed that compared with before induction, Pichia pastoris GS115 transformed with pPICZaA-Cq-TRP1 recombinant plasmid could express recombinant protein after induction, the size was about 18kDa, and the protein expression level of methanol induction 24h was significantly higher than that of 12h (see figure 2 ).
Embodiment 3
[0058] Example 3 Purification of the expression product of pPICZaA-Cq-TRP1 recombinant plasmid after methanol induction in Pichia pastoris GS115
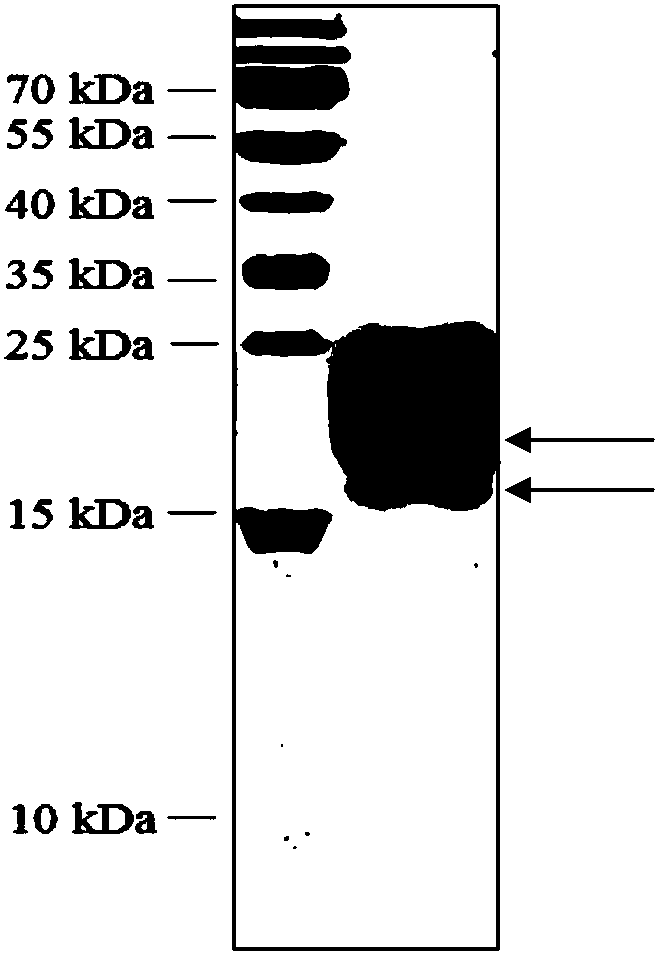
[0059] Purify the Cq-TRP1 recombinant protein by affinity chromatography, induce the expression of a large number of positive recombinant Pichia pastoris GS115 strains, remove the bacteria by centrifugation (4°C, 12000rpm centrifugation for 30min), and collect 1L of the medium supernatant in dialysate (50mM Phosphate buffered saline, 50mM NaCl) in dialysis three times (dialysis 12h each time), obtain the column sample. Then, the dialyzed protein was subjected to affinity chromatography using a metal chromatographic column. Collect the elution peak components and analyze by SDS-PAGE electrophoresis (see image 3 ) shows two bands, one is about 18kDa and the other is about 16kDa. According to mass spectrometry, the two bands of 18kDa and 16kDa are the rCq-TRP1 protein of the red claw crayfish.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com