Application of cq-ns1abp gene and its protein antiviral activity for inhibiting wssv infection
A cq-ns1abp, gene sequence technology, applied in antiviral agents, applications, genetic engineering and other directions, can solve problems such as unclear, achieve the effect of inhibiting infection replication and strong anti-WSSV infection activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 Construction of the anti-WSSV infection regulatory factor Cq-Ns1abp prokaryotic expression vector of red claw crayfish
[0043] Design and amplify the protein encoding the two important domains (BTB and Kelch) of the Cq-Ns1abp (cDNA) gene and the full-length protein containing the ORF box: Cq-Ns1abp-BTB specific upstream primer BTB-F1 and downstream primer BTB-R1, Cq-Ns1abp-Kelch specific upstream primer Kelch-F1 and downstream primer Kelch-R1, Cq-Ns1abp-Full-length specific upstream primer Ns1abp-F1 and downstream primer Ns1abp-R1. Add an EcoR I restriction site to the 5′ end of each upstream primer; add a Not I restriction site and a 6*His tag to the 3′ end of the downstream primer, so that the recombinant protein is simultaneously equipped with MBP and 6*His tags, for further purification. The corresponding domain regions were then amplified by PCR.
[0044] Cq-Ns1abp-BTB upstream primer BTB-F1:
[0045] 5'-CCGGAATTCTGCGATGTCATCCTCCAGGT-3',
[0046] Cq-...
Embodiment 2
[0058] Example 2 Induced expression of recombinant expression vector pMal-C2x-Cq-Ns1abp in Escherichia coli BL21 (DE3)
[0059] 1. Induced expression:
[0060] 1) Transform the constructed recombinant expression vector into BL21(DE3), and spread it on the LB plate containing ampicillin (Amp+) resistance.
[0061] 2) Pick a monoclonal colony, inoculate it in 5 ml of LB medium containing Amp+, and culture it on a shaking table at 37° C. at 200 rpm for 12 hours.
[0062] 3) Inoculate into 100ml LB medium containing Amp+ at a ratio of 1:100, culture at 37°C, 200rpm shaker until OD600 is 0.3 to 0.5.
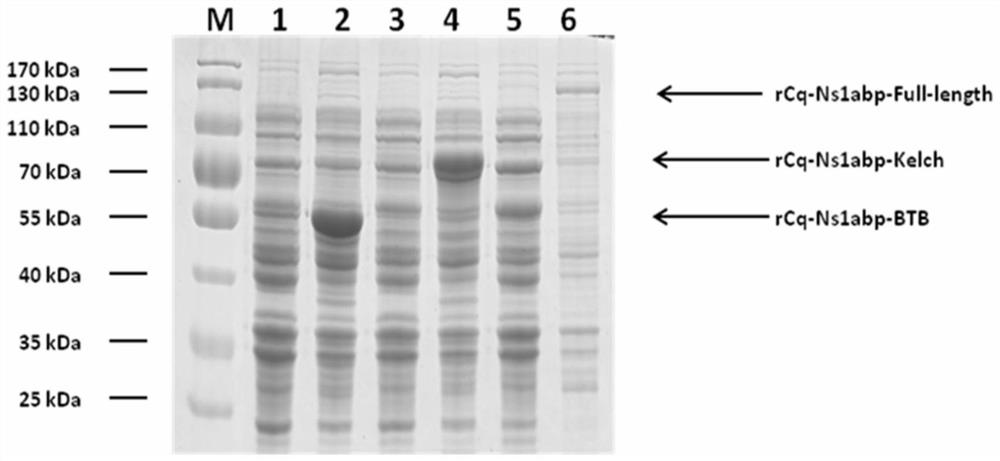
[0063] 4) Add IPTG to a final concentration of 0.1 mM, and induce at 16° C. at 150 rpm for 20 h.
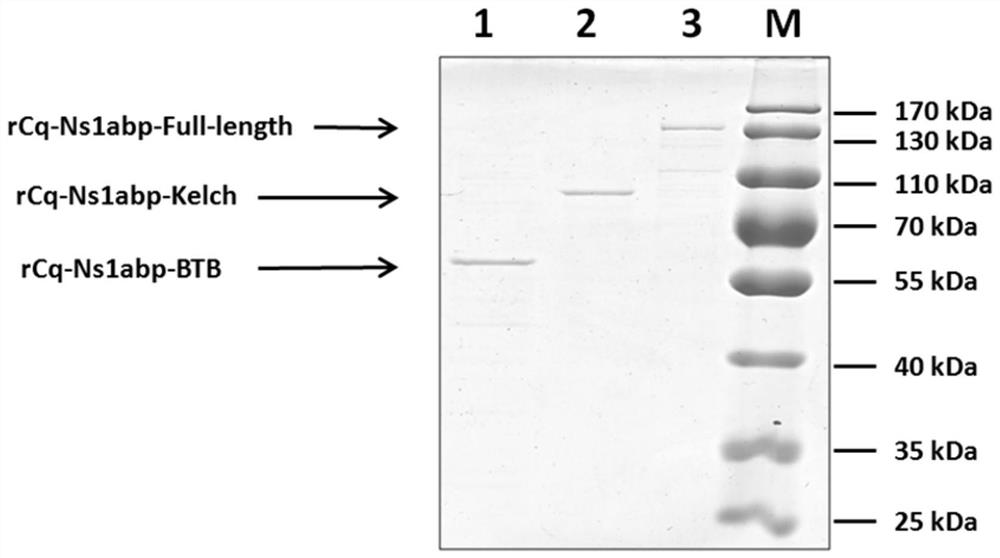
[0064] The results show that see figure 1 (2, 4, 6 swimming lanes), after IPTG induction, obvious induction bands can be detected in the bacteria, and the sizes are about 55kDa, 70kDa, and 130kDa, indicating that a higher proportion of expression products can be obtained under this co...
Embodiment 3
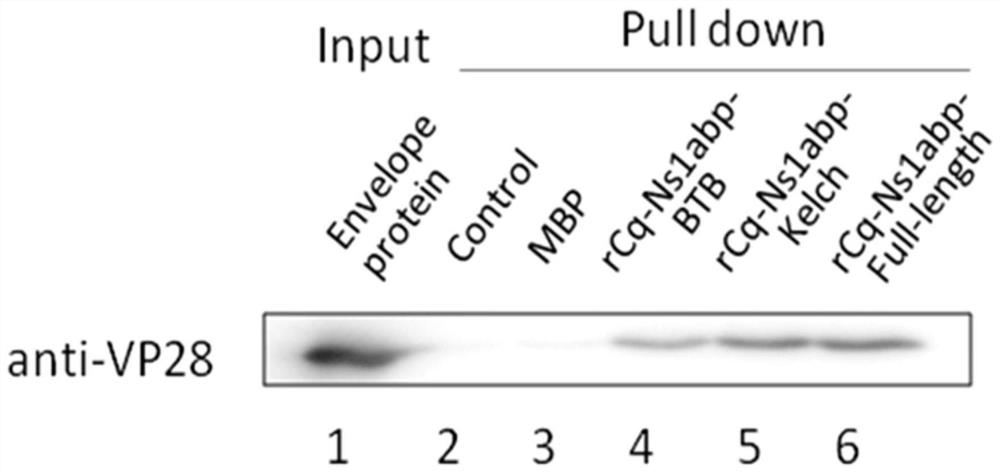
[0075] Example 3 Use of pull down experiments to demonstrate the recognition and binding of rCq-Ns1abp recombinant protein to WSSV
[0076]Dilute 2ug of recombinant protein (MBP, rCq-Ns1abp-BTB, rCq-Ns1abp-Kelch, and rCq-Ns1abp-Full-length, without any protein in the control group) and 5ug of WSSV envelope protein in 1ml of PBS solution, add 20ul of Dextrin beads that can specifically bind to MBP-tagged proteins were incubated at 4°C with rotation for 4h. After incubation, centrifuge at 500 g for 3 min, and carefully remove the supernatant. Resuspend and wash with 1ml pre-cooled PBS, centrifuge at 500g for 3min, repeat 5 times. Finally, the binding protein on the beads was eluted with 10mM maltose, added SDS sample buffer, boiled for 10min to denature, placed in 12% SDS-PAGE gel for electrophoresis, and Western Blot was used to detect recombinant protein and WSSV envelope protein Binding of VP28. The result is as image 3 , in the control group without adding protein and a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com