Bispecific antibody fitc×cd3 and its preparation method and application
A bispecific antibody and specific technology, applied in the field of bispecific antibody FITC×CD3 and its preparation, can solve the problems of poor stability, low yield, cumbersome purification steps, etc., and achieve high binding capacity, good stability, broad The effect of the application foreground
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
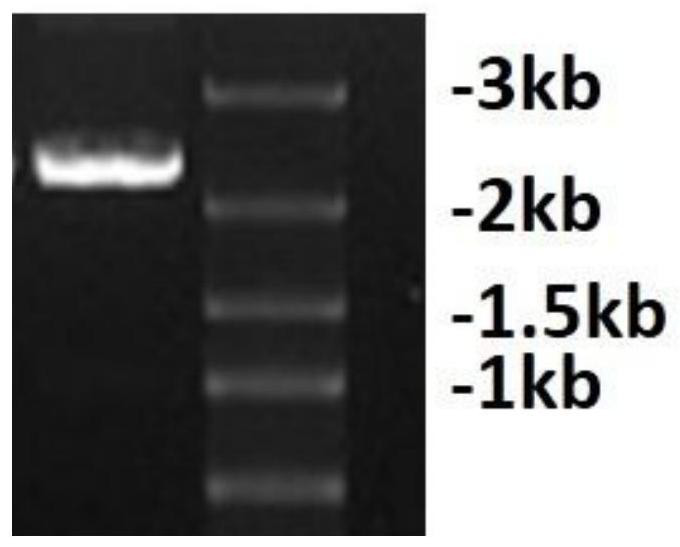
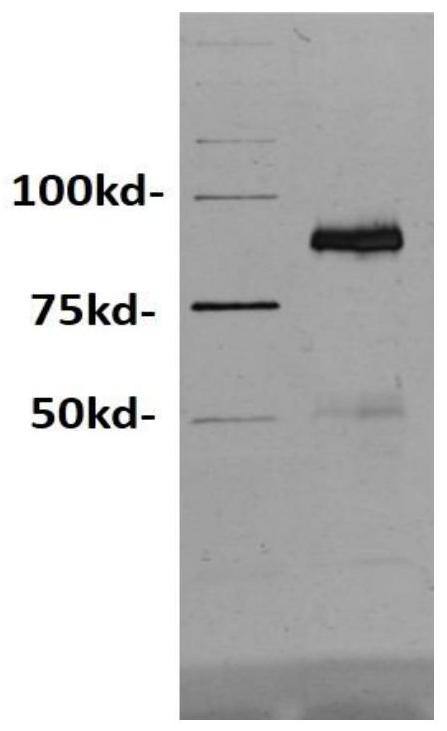
[0034] 1. Design of bispecific antibodies
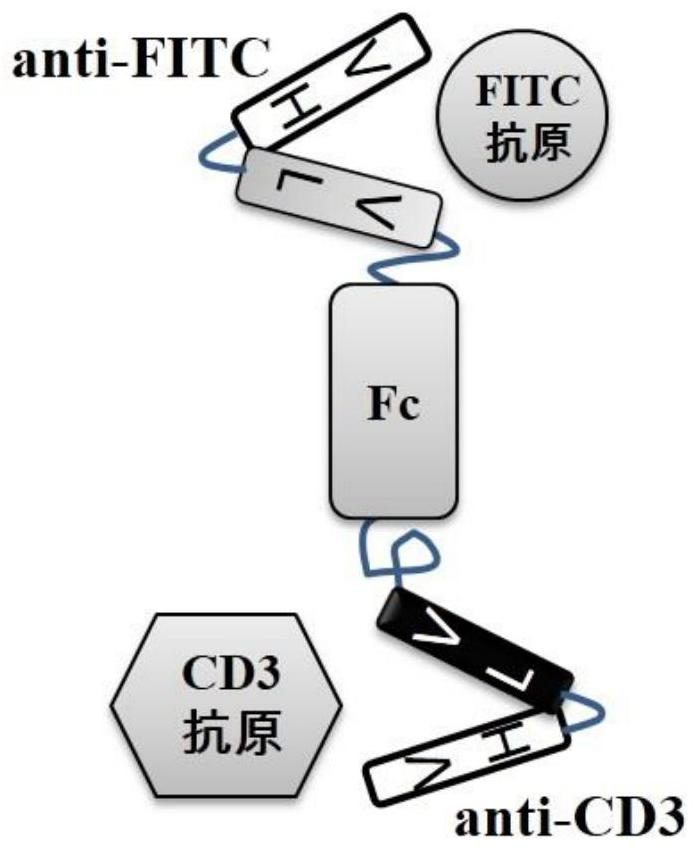
[0035] The bispecific antibody targeting FITC and CD3 is named FITC×CD3, and the structure of the antibody can be found in figure 1 , one of the monovalent antigens is an anti-FITC heavy chain-light chain pair (SEQ ID NO: 1), and the other monovalent antigen is an anti-CD3 heavy chain-light chain pair (SEQ ID NO: 2), and the sequence refers to a monoclonal antibody The sequence of L2K (refer to US20070123479 sequence number 2), the two monovalent antigens are connected by the Fc segment of human immunoglobulin 1 (SEQ ID NO: 3), and the Fc segment is connected to the anti-CD3 heavy chain-light chain A flexible linker sequence (SEQ ID NO: 4) was added between them. In order to enable FITC×CD3 to be expressed in Chinese hamster ovary cells (CHO) cells and secreted into the medium, the leader peptide sequence of the mouse immunoglobulin κ light chain was selected as the secretion signal peptide. The signal peptide is directly linked to...
Embodiment 2
[0061] Pharmacodynamic study of bispecific antibody against tumor
[0062] Mix equal volumes of phosphate buffered saline (PBS) and peripheral blood of healthy people, add equal volumes of lymphocyte separation medium, centrifuge at room temperature for 30 minutes at 2500 rpm. Take the middle buffy coat, add 10 times the volume of PBS to wash away the lymphocyte separation solution, repeat twice, count and resuspend in RPMI 1640 complete medium to obtain peripheral blood mononuclear cells (PBMC). Cell viability >95% was confirmed by trypan blue staining and cell counts were performed. Separated PBMC cells were added to the cultured tumor cell culture medium at the same time, so that the ratio of PBMC cells to tumor cells was 5:1, and the mixed cultured cells were divided into 6 groups. Group 1 was regarded as a negative group without drug addition, and group 1 2 Add 1 μg of FITC-modified antibody bevacizumab, add 1 μg of the bispecific antibody of the present invention and 1 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com