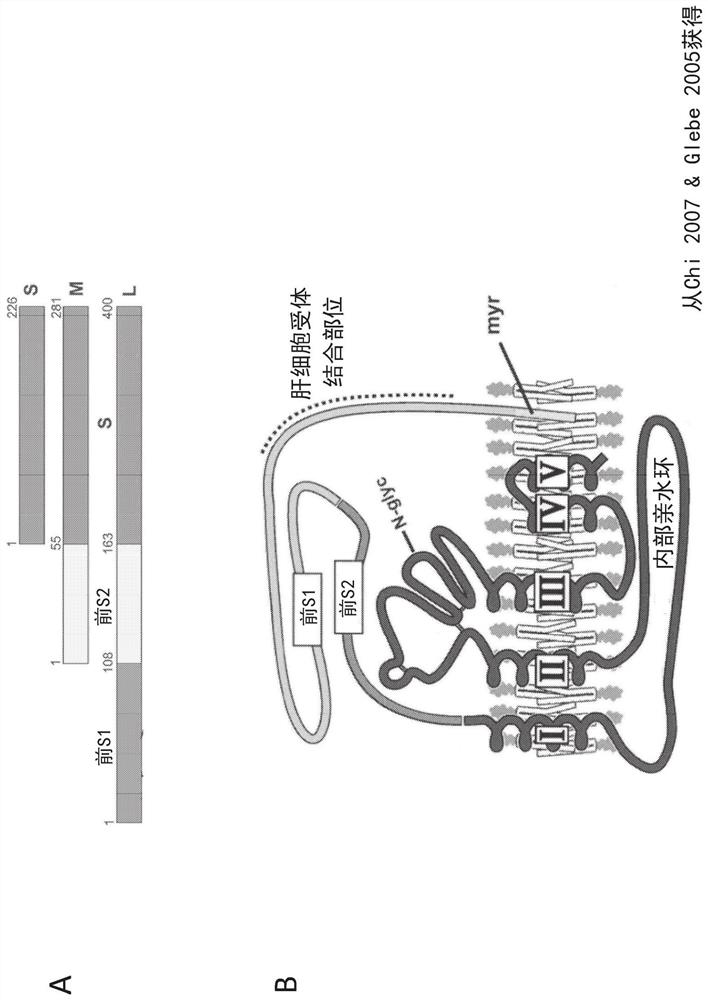
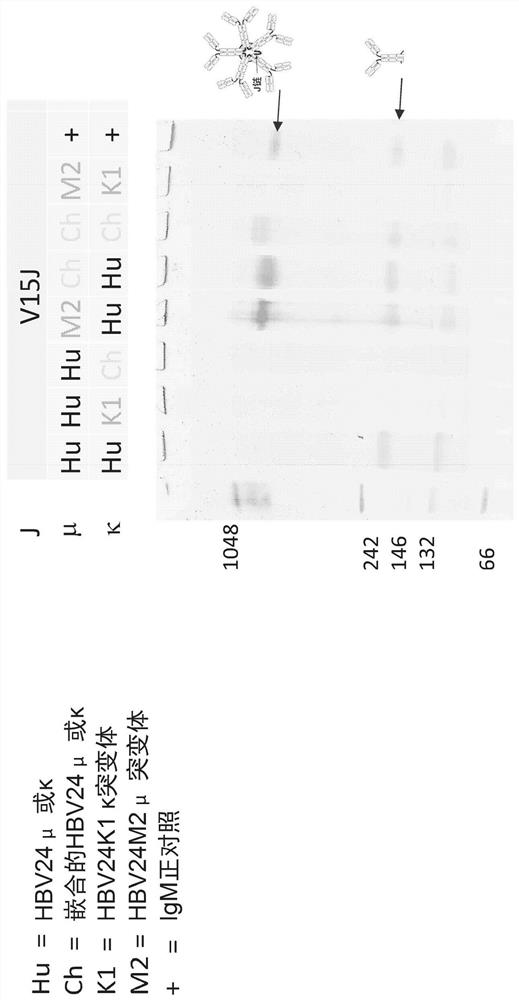
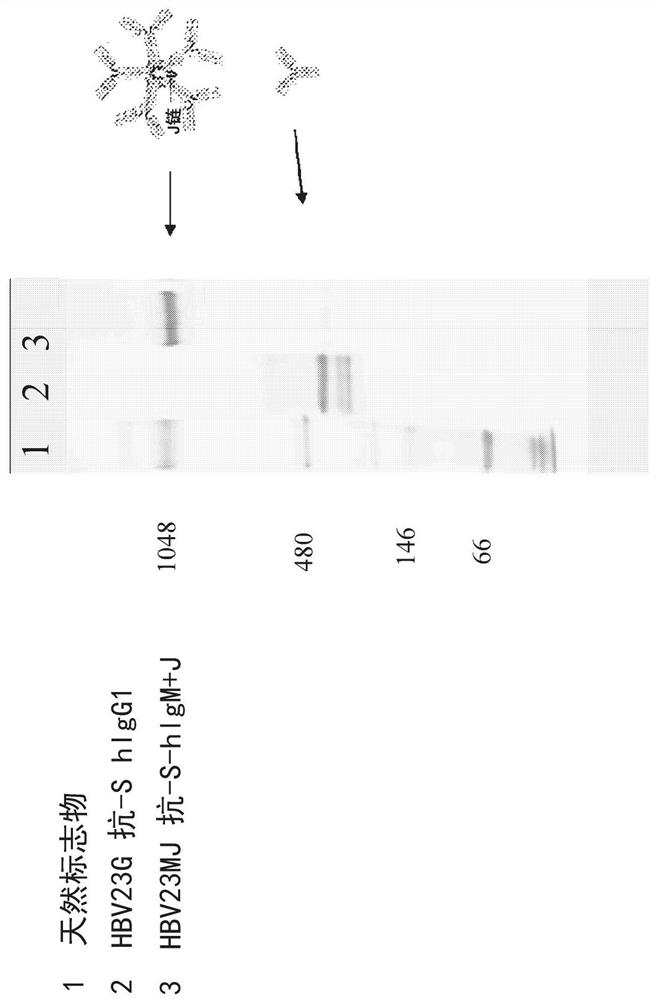
Multivalent hepatitis B virus antigen-binding molecule and application thereof
A technology that combines molecules and antigens, applied in antiviral agents, antibody medical ingredients, medical preparations containing active ingredients, etc., can solve problems such as side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0055] Furthermore, "and / or", as used herein, should be deemed to specifically disclose each of the two features or components, with or without the other. Thus, the term "and / or" as used herein in the phrase "A and / or B" is intended to include "A and B," "A or B," "A" (alone), and "B (alone)." Likewise, the term "and / or" as used in phrases such as "A, B, and / or C" is intended to include various embodiments of: A, B, and C; A, B, or C; A or C; A B or C; A and C; A and B; B and C; A (alone); B (alone); and C (alone).
[0056] Unless otherwise defined, technical and scientific terms used herein have the same meaning as commonly understood by one of ordinary skill in the art to which this invention belongs. For example, Concise Dictionary of Biomedicine and Molecular Biology, Juo, Pei-Show, 2nd Edition, 2002, CRC Press; The Dictionary of Cell and Molecular Biology (The Dictionary of Cell and Molecular Biology), 3rd ed., 1999, Academic Press; and Oxford Dictionary of Biochemistry...
Embodiment 1
[0275] Example 1: Surface antigen preparation
[0276] Surface antigens can be prepared as previously reported. (See, eg, Short et al., J. Mol. Biol. (2009) 390, 135-141). In one method, HBsAg is isolated from the blood of HBV carriers. Frozen serum containing HBV can be obtained, for example, from Diagnostics, Development and Research Division, National Blood Service, Colindale Centre, London, UK. Each package was then thawed and centrifuged (3700 x g, 20 min), the supernatant was layered in 0.5 ml of 20% sucrose TBS (in a 5.1 ml tube), and centrifuged (266,000 x g, 30 min). Particles were resuspended in 20 nM Tris chloride (pH 7.4) and 140 mM NaCl (Tris buffered saline) (wet at 4°C overnight to soften), pooled and equilibrated with CsCl (0.22 g / ml initial concentration) by centrifugation (266,000 x g, 72 hours). Fractions (250 [mu]l) were removed from the gradient and monitored by electron microscopy and DNA extraction, analyzing hepatitis B DNA. Fractions containing th...
Embodiment 2
[0277] Example 2: HBV capsid and virion isolation
[0278] Methods of isolation of capsids and virions are well known in the art and can be carried out by various known methods. (See, eg, Dryden, Molecular Cell, 22:843-850, Jun 23, 2006, Supplement). In one method of capsid isolation, freshly dissected whole livers are perfused with saline solution. The liver is then homogenized in lysis buffer (eg, 0.25M sucrose, 1 mM MgCl 2 , 5 mM Tris (pH 7.4)) and supplemented with complete protease inhibitor (Roche Applied Science, Indianapolis, IN). , the supernatant of each homogenate was obtained by centrifugation in a SW40 rotor (SW40rotor) at 4°C, 11,000 rpm for 15 minutes. Aliquots of the supernatant were taken for HBV-specific Southern blot analysis. The residue was then layered on a 30% sucrose pad (0.73M sucrose in phosphate buffered saline (PBS)) and the viral particles were pelleted by centrifugation in a SW40 rotor at 40,000 rpm for 5 hours at 4°C. Thereafter, the particl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com