Application of neuraminidase and inhibitor in preparing medicine for treating pulmonary fibrosis and renal fibrosis
A technology of neuraminidase and pulmonary fibrosis, applied in the field of biomedicine, can solve the problems of undiscovered neuraminidase correlation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1: Relationship between neuraminidase and its inhibitors and pulmonary fibrosis
[0020] 1. Experimental method
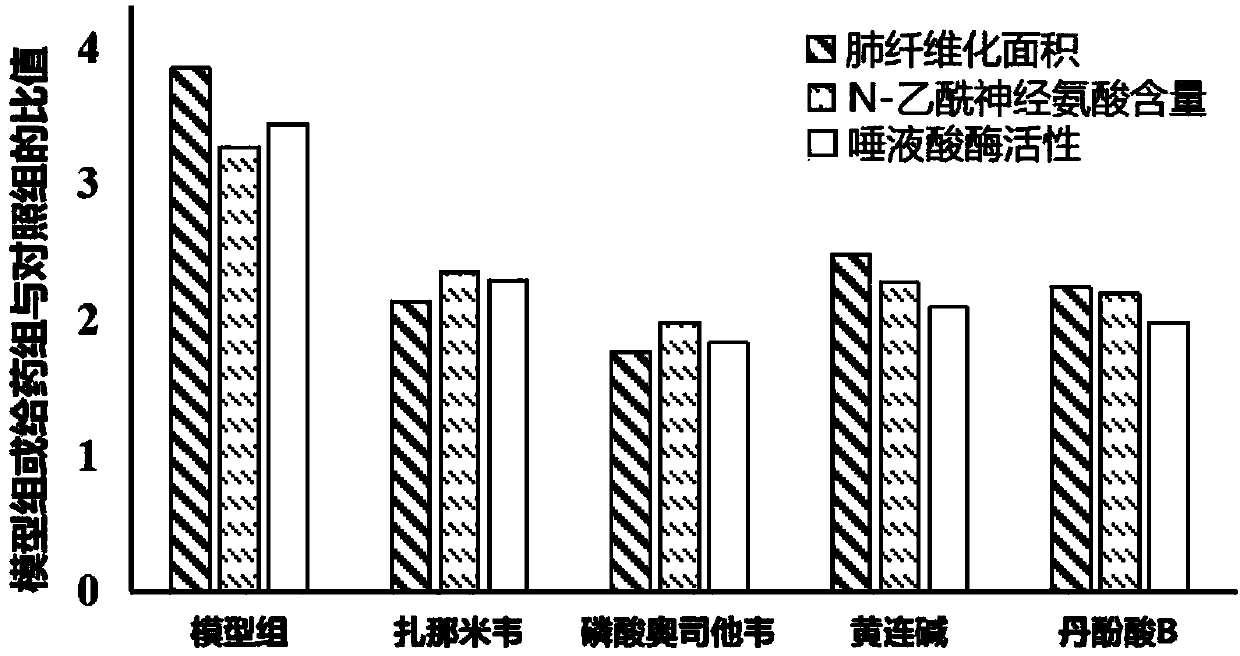
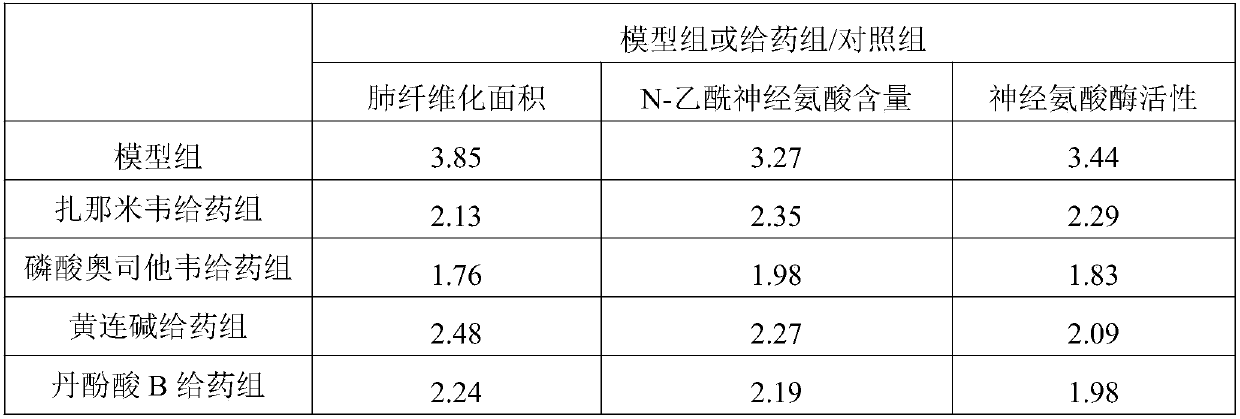
[0021] Grouping and administration: SPF male C57BL / 6 mice (weight 20-24g) were adaptively reared for 3-5 days and then randomly divided into sham operation group (control group), model group, zanamivir, oseltami phosphate Wei, coptisine, salvianolic acid B administration group. Except for the control group, which was injected with equal volume of sterile saline, the other groups were injected with BLM (5 mg / kg) through the trachea to establish models. After 2 hours, the mice in the administration group were given 0.2 mg / kg / d zanamivir (i.v.), 5 mg / kg / d oseltamivir phosphate (p.o.), 40 mg / kg / d coptisine (p.o.), 40 mg / kg / d salvianolic acid B (p.o.), the model group and the control group were given an equal volume of vehicle 0.5% CMC-Na by gavage for 4 weeks. 2 hours after the last administration, the patients were sacrificed by exsanguination of the...
Embodiment 2
[0031] Example 2: Relationship between neuraminidase and its inhibitors and renal fibrosis
[0032] 1. Experimental method
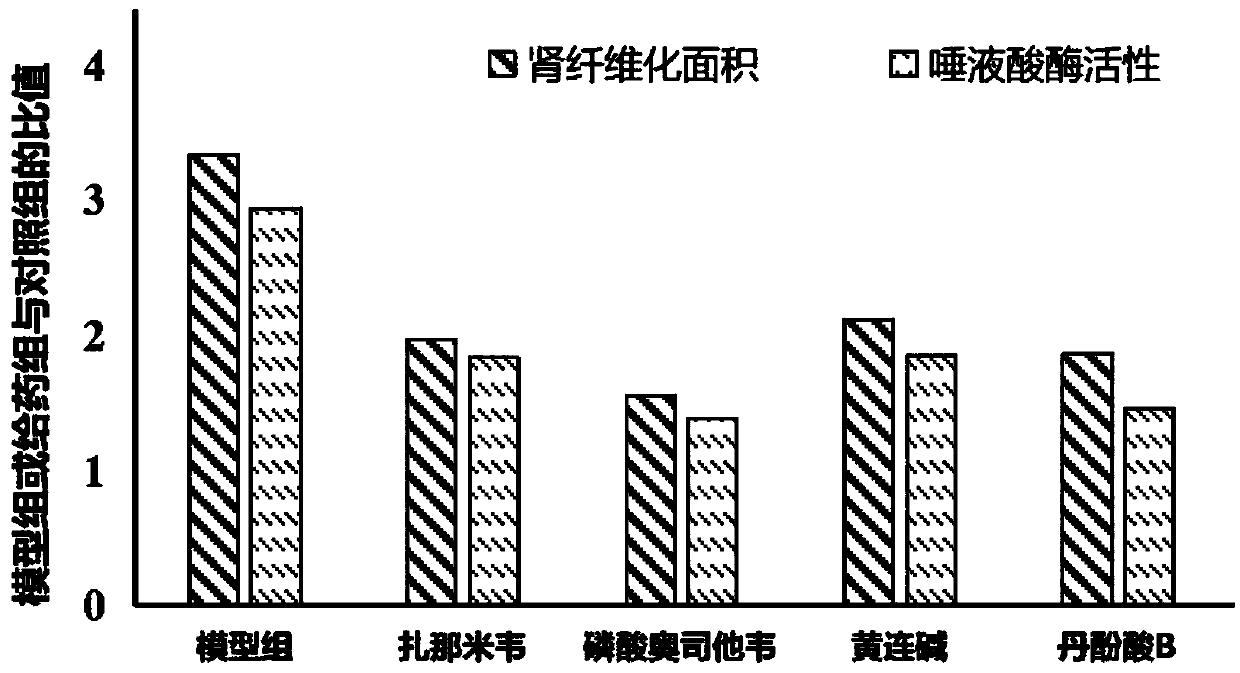
[0033] Grouping and administration: Male clean-grade Wistar rats (body weight 160-180 g) were adaptively reared for 3-5 days and then randomly divided into sham operation group (control group), model group and zanamivir, oseltamivir phosphate, Coptis alkaloid, salvianolic acid B administration group. Except for the control group, the other groups of rats were modeled. The modeling method is as follows: after the rats were anesthetized by ip 10% chloral hydrate (4ml / kg), the rats were fixed in the right lateral position on the operating table, the skin was routinely prepared for disinfection, the left abdominal incision was made, and the rats were separated layer by layer. The tissue was extended to the peritoneum, the left ureter was exposed and freed, and the upper 1 / 3 and the middle 1 / 3 of the ureter were ligated with silk threads, and then the urete...
Embodiment 3
[0043] A commercially available neuraminidase inhibitor screening kit P0309 (Beyotime, Beyotime) was used to test the inhibitory activity of salvianolic acid B in vitro, and the positive control drug was oseltamivir phosphate. Add 70 μL of buffer and 10 μL of neuraminidase solution to each well of the 96-well plate, then add 10 μL of different concentrations of the solution to be tested, shake and mix, incubate at 37°C for 5 min, add 10 μL of the solution containing the fluorescent substrate, and shake to mix. After 30min incubation at 37°C, fluorescence measurement was performed, where the excitation wavelength was 322 nm and the emission wavelength was 450 nm. According to the fluorescence readings, the inhibition rates of different test solutions were calculated, and the IC50 values of the positive control drugs oseltamivir phosphate and salvianolic acid B were further obtained. The results are shown in Table 3.
[0044] Table 3 IC50 values of positive control drugs os...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com