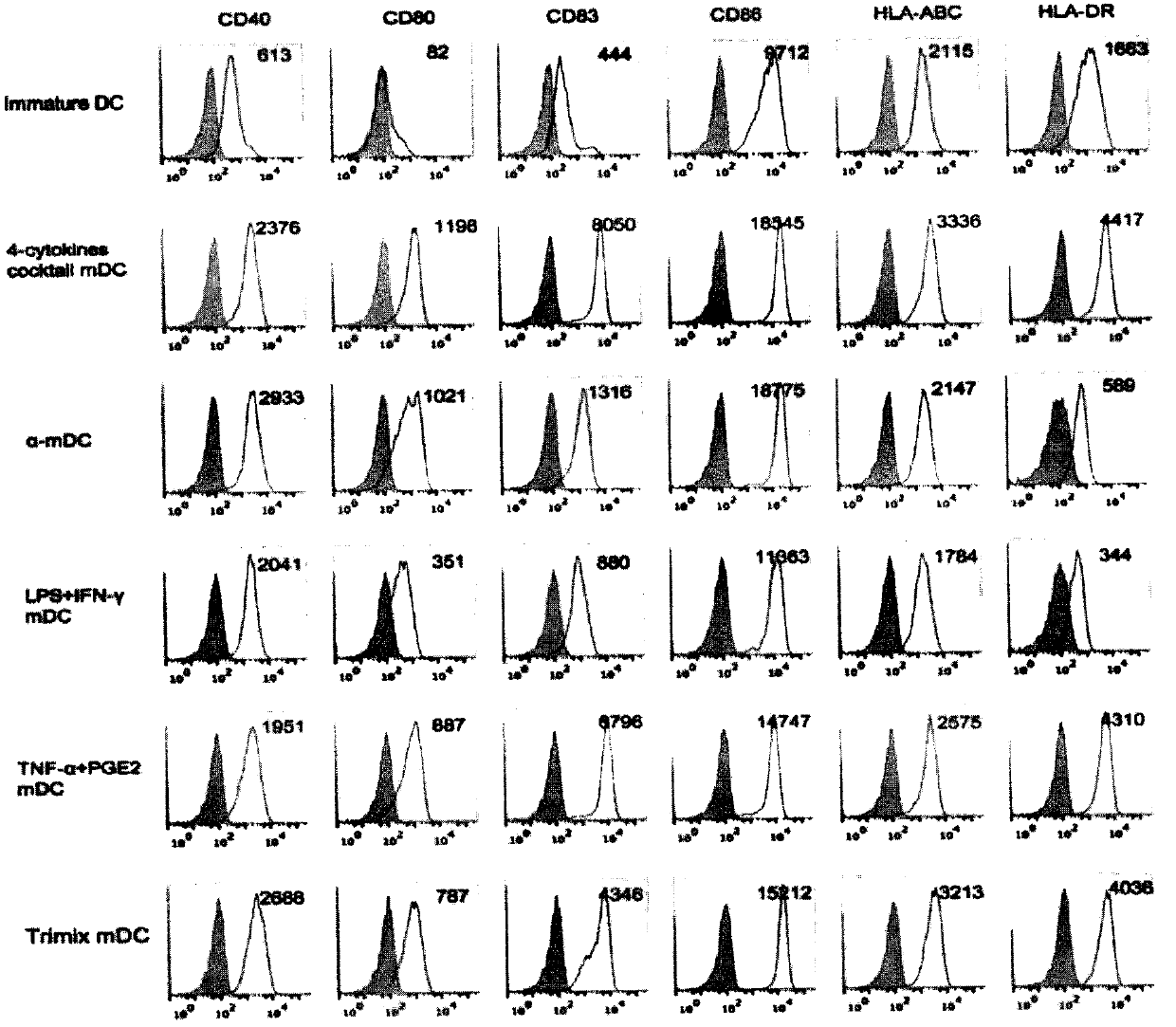
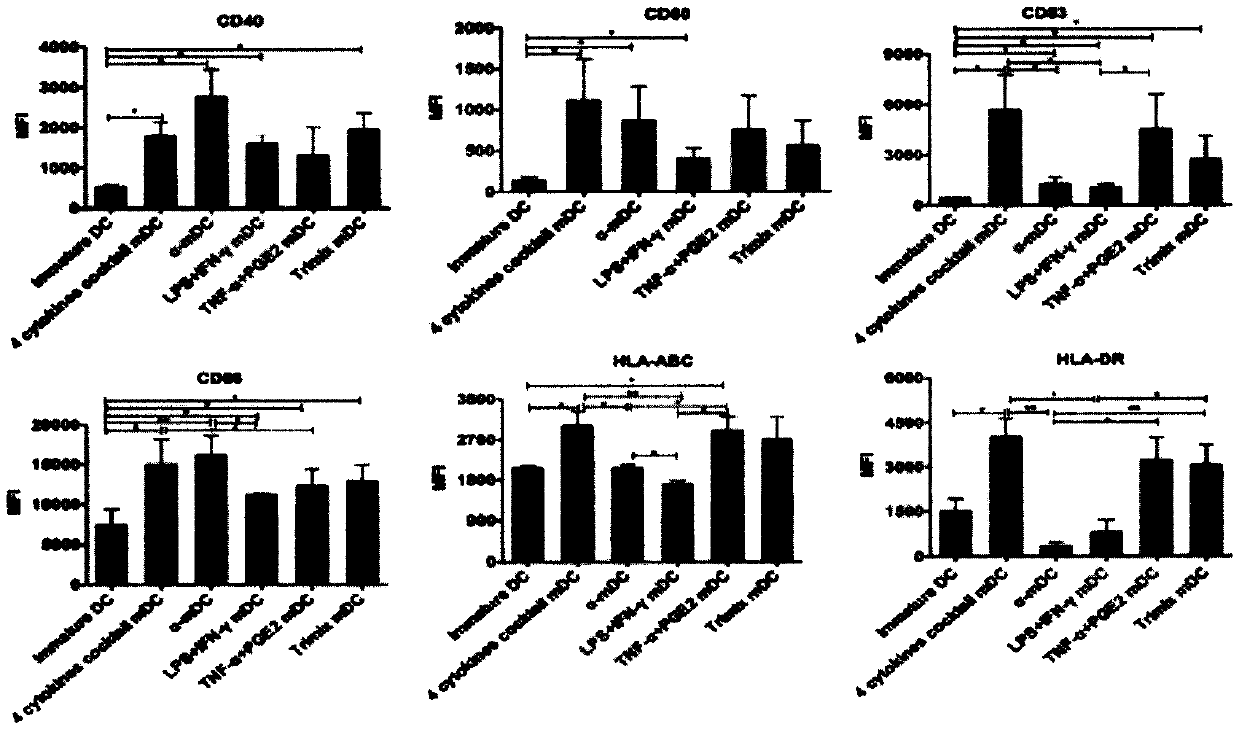
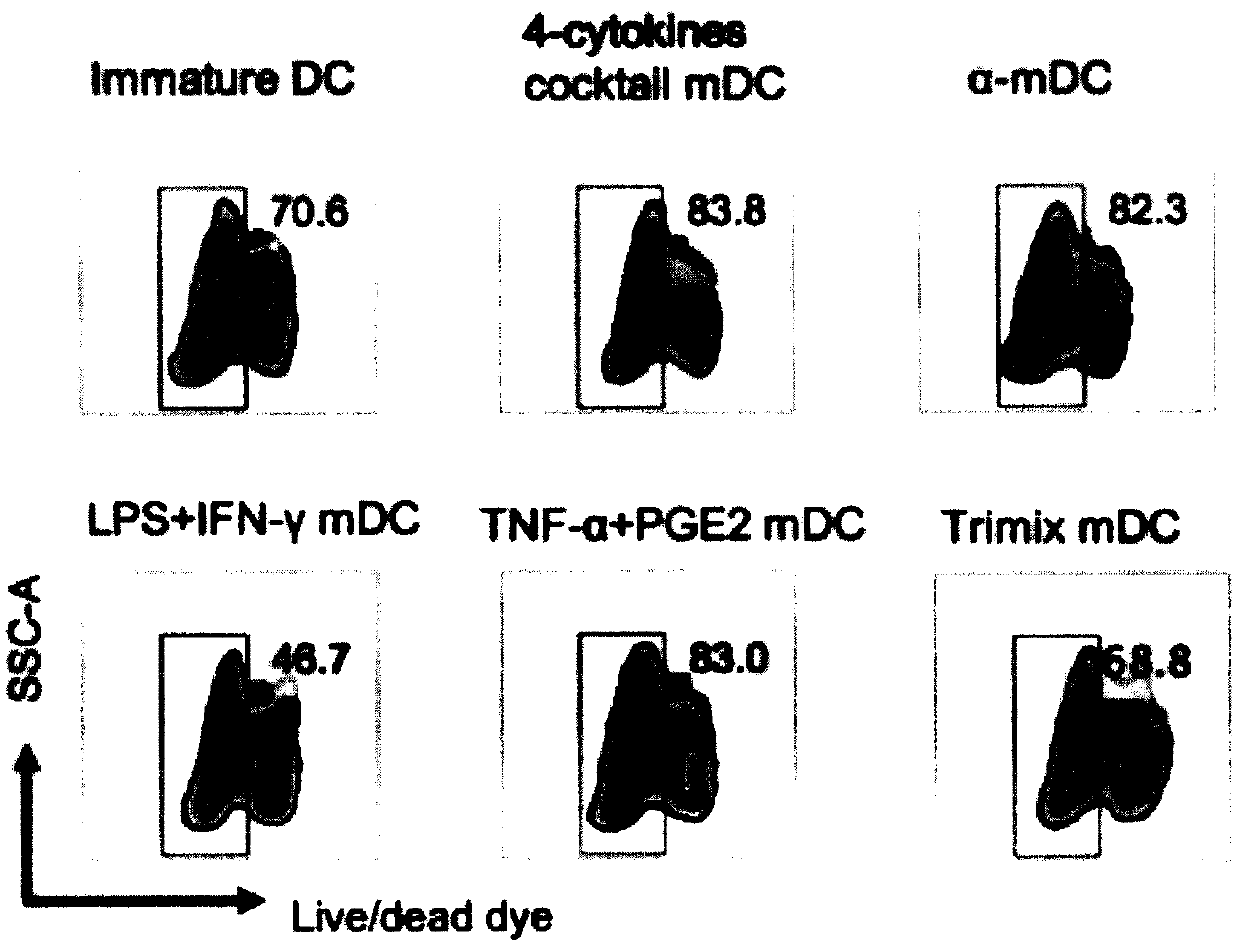
Novel preparation method of dendritic cell (DC) vaccine
A technology of dendritic cells and vaccines, applied in the field of cellular immunology, can solve the problem of reducing DC cells, achieve the effect of increasing cell survival time and cell survival rate, and improving tumor killing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] In vitro transcription and synthesis of Bcl-2, IL-12p70mRNA
[0050] 1. Construct plasmids containing Bcl-2 and IL-12p70 respectively, the nucleotide sequences of both are obtained from GeneBank;
[0051] 2. Design primers according to the Bcl-2 and IL-12p70 gene sequences, clone the fragments of the two by PCR, and connect the cloned genes to the psp73 vector;
[0052] 3. Treat the psp73 vector connected with Bcl-2 and IL-12p70 genes with speI endonuclease to linearize it;
[0053] 4. Synthesis of Bcl-2 and IL-12p70 mRNA by in vitro transcription
Embodiment 2
[0055] Preparation and detection of human dendritic cell vaccine
[0056] 1. Collect 100ml of peripheral blood from the patient, dilute blood cells with PBS at a ratio of 2:1, add Ficoll-Paque to the diluted blood at a ratio of 1:2 into a centrifuge tube, and centrifuge at 700g for 20 minutes. After centrifugation, carefully aspirate the buffy coat cells, and wash the cells twice with calcium-magnesium-free PBS or HBSS at 500g for 10 minutes and 300g for 10 minutes, respectively.
[0057] 2. Resuspend the mononuclear cells isolated above in 10ml of AIM-V or X-VIVO-15 medium, transfer the cells into a T175cm culture flask, and supplement the medium to 30ml. The culture flask was placed in a 37°C, 5% carbon dioxide incubator for 2 hours to adhere to the wall.
[0058] 3. Gently tap the culture bottle to re-suspend the non-adhered cells, collect the non-adhered cells into a centrifuge tube; add PBS to the bottle, gently shake the culture bottle to wash away the non-adhered cells...
Embodiment 3
[0069] Tumor antigen-specific T cell responses induced by DC cells in vitro
[0070] 1. One day before DC cell electroporation, thaw the frozen non-adherent lymphocytes, resuspend the cells in 15ml of AIM-V or X-VIVO-15 medium, transfer them to a T75 culture flask, and place at 37°C, 5% Recover overnight in a carbon dioxide incubator.
[0071] 2. On the second day, DC cells and T cells 2 hours after electroporation were mixed and cultured at a ratio of 1:10. The remaining DCs were then frozen.
[0072] 3. On the third day of co-cultivation, IL-2 was added with a final concentration of 50U / ml, and half of the cells were replaced with fresh medium containing 50U / ml IL-2 every 2 days.
[0073] 4. After 7-10 days of co-culture, DC cells were revived and counted. At the same time, co-cultured T cells were collected and counted.
[0074] 5. Mix and co-culture the recovered DC cells with the collected T cells at a ratio of 1:10.
[0075] 6. After re-stimulating the T cells in ste...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com