Preparation method of high-efficiency killer cell preparation with double blocking CTL of immune checkpoint
A technology of immune detection points and killing cells, which is applied in the direction of blood/immune system cells, cell culture active agents, biochemical equipment and methods, etc., can solve the problems of low clinical objective effective rate and low tumor killing efficiency, and achieve slowing down of T Effects of cell depletion, improvement of tumor killing effect, and improvement of clinical objective effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
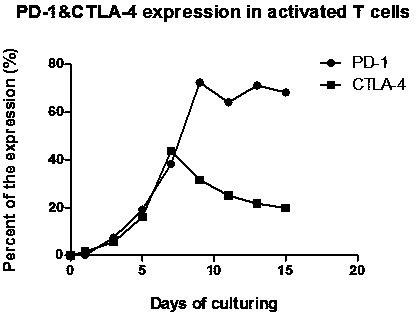
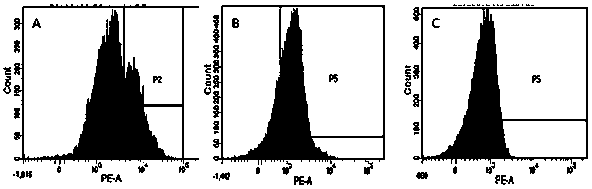
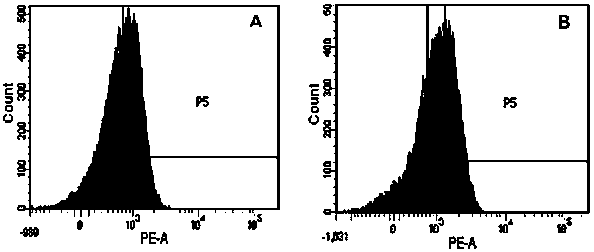
[0031] In this example 1, PD-1 and CTLA-4 antibodies were used to block the inhibitory signal of CTL preparations, and a high-efficiency double-deleted CTL preparation was prepared. At the same time, the ligands PD-L1 and CTLA-4 of PD-1 on the surface of tumor cells were detected. The expression characteristics of ligands B7-1 and 2 (CD80, CD86), the specific steps are as follows:
[0032] 1) Obtaining monocytes:
[0033] Collect 100ml of peripheral blood mononuclear cells (or collect hematopoietic stem cells from umbilical cord blood to prepare allogeneic DC) from tumor patients by venous extraction or blood component separation machine, collect whole blood cells by centrifugation, transfer into lymphocyte separation medium, and centrifuge at 2000rpm×15 with a horizontal rotor Minutes, aspirate the middle buffy coat to collect monocytic cells.
[0034] 2) DC and T cell sorting:
[0035] The monocytes collected above were transferred into culture flasks containing serum-free...
Embodiment 2
[0047] Example 2 is the application of Dual-block CTL effector cell preparations to conduct laboratory killing experiments (in vitro) and animal experiments (in vivo) on target cells (breast cancer). At the same time, DC-CIK and antigen-specific CTL Effector cells were controlled to verify the advantage of Dual-block CTL in killing efficiency.
[0048] 1. In vitro killing experiment research:
[0049] 1) Effector cells: Dual-block CTL (experimental group), T cells (control 1), DC-CIK (control 2), CTL (control 3).
[0050] 2) Target cells: breast cancer cell line (MDA-SB435S).
[0051] 3) Experimental groups: Dual-block CTL (experimental group), T cells (control 1), DC-CIK (control 2), CTL (control 3), NS (negative control).
[0052] 4) In vitro killing and cytokine secretion experiments:
[0053] For cytotoxicity analysis, inoculate MDA-SB435S target cells in a 96-well plate, add effector cells Dual-block CTL, CTL, DC-CIK, T and NS, respectively, 3 wells in each group, co-c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com