Streptococcus pneumoniae protein vaccine and preparation method thereof
A technology for protein vaccines and pneumococci, which is applied in the direction of antibacterial drugs, bacterial antigen components, carrier-bound antigen/hapten components, etc. It can solve the problems of easily blocked absorption, poor immune response, and inability to produce immune memory, so as to improve antibody production. Concentration and seroconversion rate, the effect of reducing the probability of inoculation failure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] 1. Lectin preparation
[0053]Take 500 grams of fresh pinellia tubers from Shanghai University of Traditional Chinese Medicine, wash and grind, centrifuge at 6000rpm / min for 10min, and the supernatant is fractionally purified by ammonium sulfate with a saturation degree of 20% to 95%, and then collect 60% to 95% saturated sulfuric acid Ammonium precipitates PTA, and the active components are subjected to mannose-Sepharose gel chromatography in stages to obtain Pinellia lectin protein solution for future use. Alternatively, the lectin can be cloned into Escherichia coli for expression and isolated and purified for future use.
[0054] 2. Preparation of pneumococcal capsular saccharide
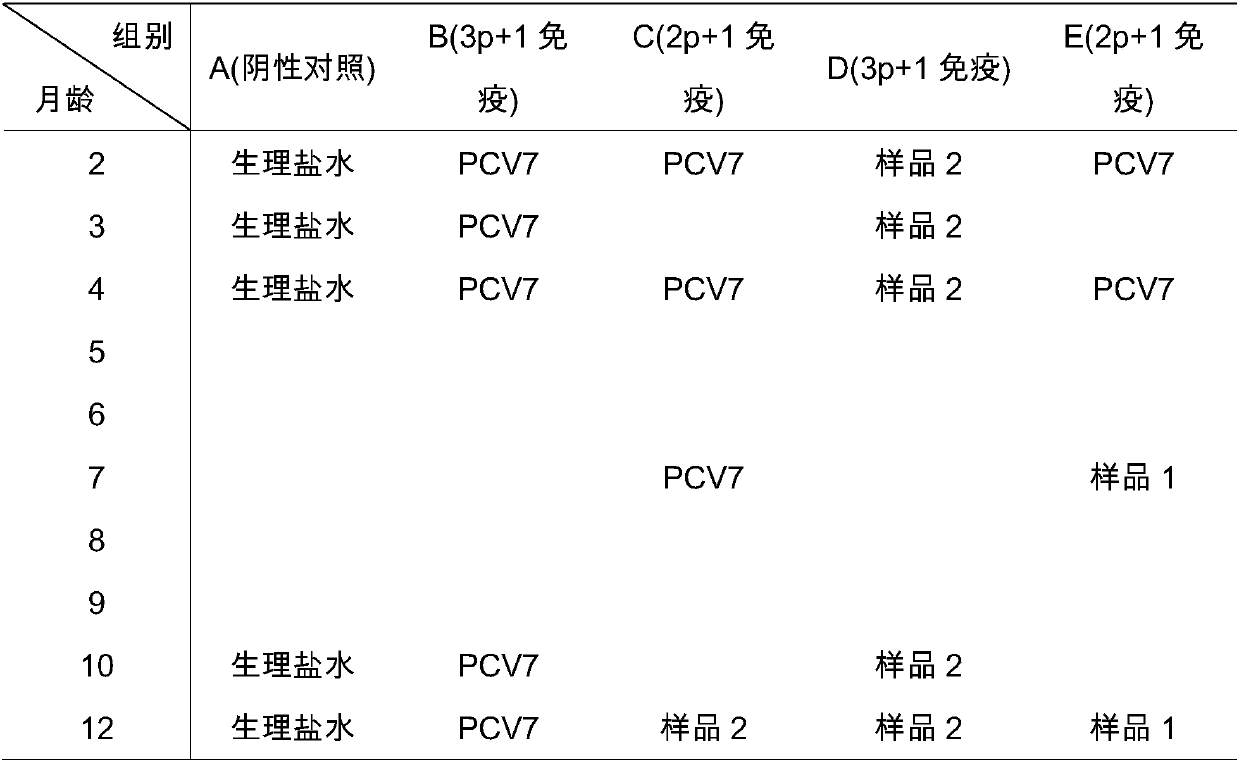
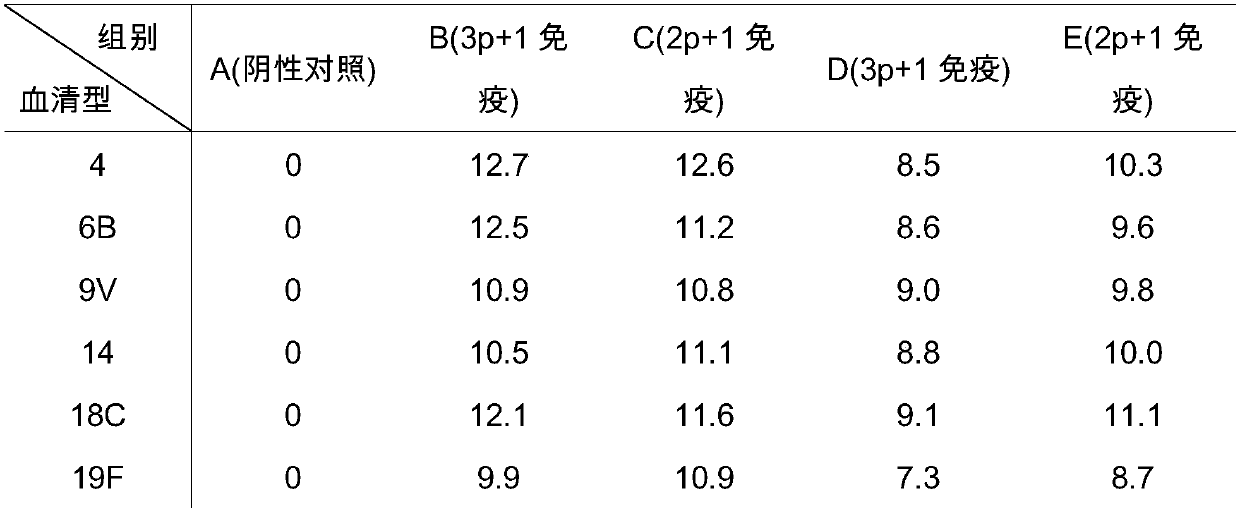
[0055] 1. Select seven serotypes (4, 6B, 9V, 14, 18C, 19F and 23F) for culture of pneumococci;
[0056] 2. Respectively purify the capsular sugars with strong antigenicity in the above various serotypes of pneumococci: centrifuge to collect the supernatant after inactivation of pneumoco...
Embodiment 2
[0064] The difference between this example and Example 1 is that the lectin is the gore agglutinin detoxified from the agarose beads, and the obtained protein vaccine solution is numbered as sample 2.
Embodiment 3
[0066] The difference between this example and Example 1 is that the serotype and dosage of the vaccine capsular saccharide are 4g each of 1, 4, 5, 7F, 9V, 14, 18C, 19F and 23F, and 8g for 6B.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com