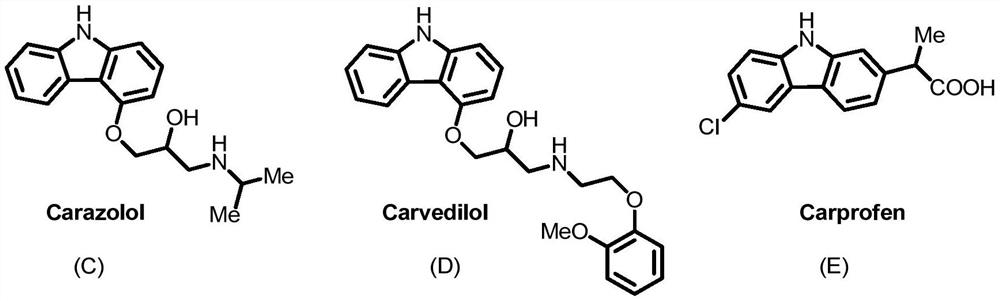
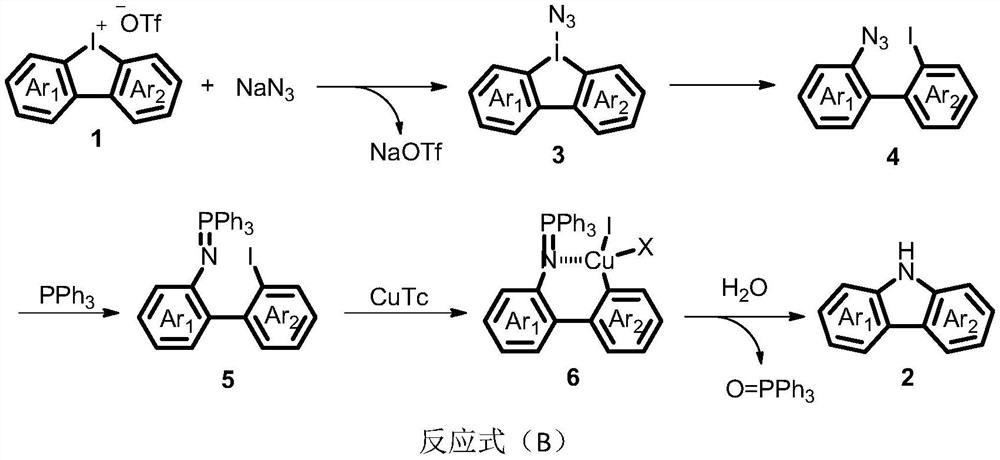
Carbazole compound and its synthesis method and application
A synthesis method and compound technology, which are applied in the preparation of non-steroidal anti-inflammatory drug carprofen, and the field of synthesis of functionalized carbazole compounds, can solve the problem of unfavorable protection and deprotection of nitrogen-containing compounds and atoms of periodic salts Problems such as poor economy, simplified reaction steps, etc., to avoid incompatibility, good yield, and simplified operation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Synthesis of Compound 2a:
[0040]
[0041] Under the protection of nitrogen, periodinium salt 1a (42.8mg, 0.1mmol), NaN 3 (7.8mg, 0.12mmol), PPh 3 (39.3mg, 0.15mmol), NaHCO 3(42.5 mg, 0.2 mmol), CuTc (1.9 mg, 0.01 mmol) and dry DMA (1 mL) were added to a dry Schlenk reaction tube. The reaction was stirred at 100°C for 12 hours, cooled to room temperature, diluted with 10 mL of water, extracted with ethyl acetate (10 mL*3), dried over anhydrous sodium sulfate, filtered, concentrated, and separated by column chromatography to obtain a colorless Oil 2a (12.6 mg, 89%). 1 H NMR (400MHz, CDCl 3 )δ8.13-8.02(m,3H),7.47-7.39(m,4H),7.28-7.20(m,2H); 13 C NMR (100MHz, CDCl 3 )δ139.5, 125.8, 123.3, 120.3, 119.4, 110.6; MS(EI) m / z=167(100).
Embodiment 2
[0043] Synthesis of compound 2b:
[0044]
[0045] Under the protection of nitrogen, periodinium salt 1b (45.8mg, 0.1mmol), NaN 3 (7.8mg, 0.12mmol), PPh 3 (39.3mg, 0.15mmol), NaHCO 3 (42.5 mg, 0.2 mmol), CuTc (1.9 mg, 0.01 mmol) and dry DMA (1 mL) were added to a dry Schlenk reaction tube. After the reaction was stirred at 100°C for 12 hours, it was lowered to room temperature, 10 mL of water was added to the system for dilution, and ethyl acetate (10 mL*3) was added for extraction, dried over anhydrous sodium sulfate, filtered, concentrated, and separated by column chromatography to obtain a white solid 2b (14.8 mg, 75%). 1 H NMR (400MHz, d 6 -DMSO) δ11.11(s, 1H), 7.97(t, J=8.6Hz, 2H), 7.41(d, J=8.0Hz, 1H), 7.27(t, J=7.6Hz, 1H), 7.10( t, J=7.3Hz, 1H), 6.96(d, J=2.1Hz, 1H), 6.76(dd, J=8.5, 2.2Hz, 1H), 3.84(s, 3H); 13 C NMR (100MHz, d 6 -DMSO)δ158.5,141.1,139.7,124.1,122.6,120.9,119.2,118.5,116.2,110.6,107.7,94.4,55.2; IR(KBr)ν3399,2924,2856,1729,1607,1460,1376,13503,...
Embodiment 3
[0047] Synthesis of compound 2c:
[0048]
[0049] Under the protection of nitrogen, periodinium salt 1c (48.5mg, 0.1mmol), NaN 3 (7.8mg, 0.12mmol), PPh 3 (39.3mg, 0.15mmol), NaHCO 3 (42.5 mg, 0.2 mmol), CuTc (1.9 mg, 0.01 mmol) and dry DMA (1 mL) were added to a dry Schlenk reaction tube. After the reaction was stirred at 100°C for 12 hours, it was lowered to room temperature, 10 mL of water was added to the system for dilution, and ethyl acetate (10 mL*3) was added for extraction, dried over anhydrous sodium sulfate, filtered, concentrated, and separated by column chromatography to obtain a white solid 2c (10.1 mg, 45%). 1 H NMR (500MHz, d 6 -acetone) δ8.22(d, J=1.7Hz, 1H), 8.01(d, J=7.8Hz, 1H), 7.97(d, J=8.4Hz, 1H), 7.46(d, J=8.1Hz, 1H), 7.35-7.28(m, 1H), 7.19(dd, J=8.4, 1.8Hz, 1H), 7.14(t, J=7.5Hz, 1H), 2.13(s, 3H); 13 C NMR (126MHz, d 6 -acetone)δ168.8,141.4,141.1,138.6,125.6,124.0,120.9,120.3,119.7,112.0,111.4,102.1,24.4; IR(KBr)ν2925,2859,1666,1601,1543,1459,1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com