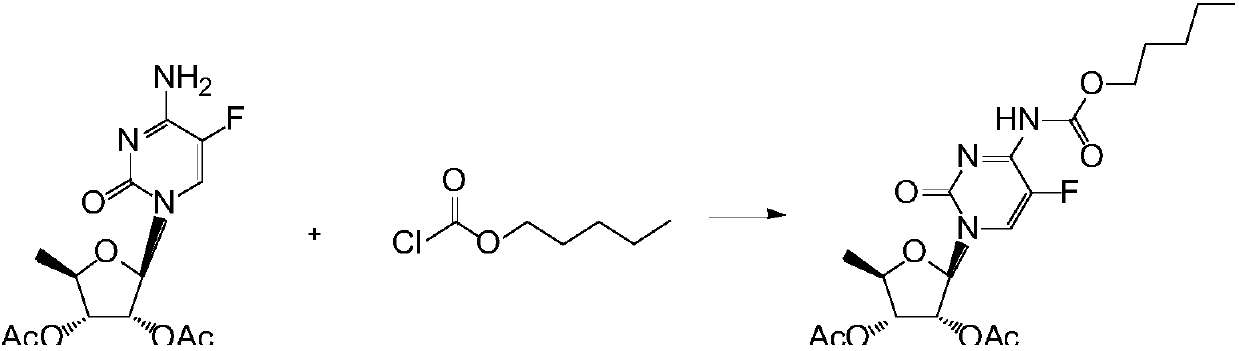
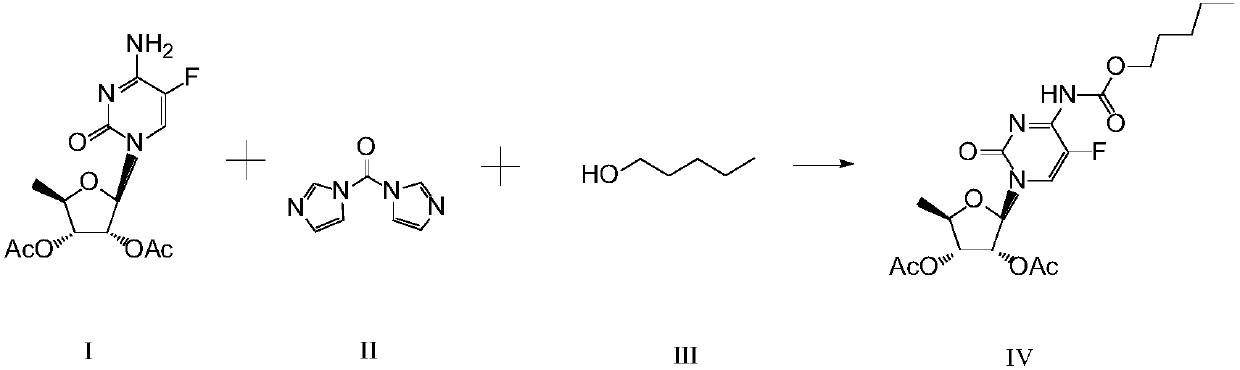
Synthesis method of capecitabine intermediate
A technology of capecitabine and synthesis method, which is applied in chemical instruments and methods, preparation of sugar derivatives, sugar derivatives, etc., can solve the problems of high market price of n-amyl chloroformate, increase of production cost, etc., and achieve favorable Industrial production, less by-products, and the effect of avoiding impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1. Add 500ml of dichloromethane, 75g of 2',3'-di-O-acetyl-5'-deoxy-5-fluorocytidine, 44.3g of carbonyldiimidazole, and 22g of n-pentanol into a 1000ml reaction flask , and react at a temperature of 20°C for 4 hours, monitor the end point of the reaction by TLC (developer, dichloromethane:methanol=20:1), add 200ml of 10% hydrochloric acid solution to the reaction solution for washing, separate the organic layer, wash with 200ml of purified water, Separate the organic layer, control the temperature of the water bath at 50°C, concentrate the organic layer to dryness under reduced pressure, add 150ml of ethyl acetate and 300ml of n-hexane to the residue and stir for 30 minutes, lower the temperature below 10°C, and start suction filtration to obtain a white solid. Dry at 65°C to obtain 92.5 g of 2',3'-di-O-acetyl-5'-deoxy-5-fluoro-N4-(n-pentyloxycarbonyl)cytidine, with a yield of 91.6% and a purity of 99.6% .
Embodiment 2
[0025] Example 2. Add 660ml of dichloromethane, 97.8g of 2',3'-di-O-acetyl-5'-deoxy-5-fluorocytidine, 56.9g of carbonyldiimidazole, and n-amyl alcohol into a 1000ml reaction flask 29.1g, reacted at 20°C for 4 hours, monitored the reaction end point by TLC (developing solvent, dichloromethane:methanol=20:1), added 250ml of 10% hydrochloric acid solution to the reaction solution for washing, separated the organic layer, and washed with 250ml of purified water Wash, separate the organic layer, control the temperature of the water bath at 55°C, concentrate the organic layer to dryness under reduced pressure, add 170ml of ethyl acetate and 340ml of n-hexane to the residue and stir for 30 minutes, lower the temperature below 10°C, and start suction filtration to obtain a white solid , controlled temperature at 70°C, dried to obtain 120.9g of 2',3'-di-O-acetyl-5'-deoxy-5-fluoro-N4-n-pentyloxycarbonyl cytidine, yield 91.5%, purity 99.6% .
Embodiment 3
[0026] Example 3. Add 600ml of dichloromethane, 84.8g of 2',3'-di-O-acetyl-5'-deoxy-5-fluorocytidine, 48.6g of carbonyldiimidazole, and n-amyl alcohol into a 1000ml reaction flask 25.2g, reacted at 20°C for 4 hours, monitored the reaction end point by TLC (developer, dichloromethane:methanol=20:1), added 220ml of 10% hydrochloric acid solution to the reaction solution for washing, separated the organic layer, and washed with 220ml of purified water Wash, separate the organic layer, control the temperature of the water bath at 60°C, concentrate the organic layer to dryness under reduced pressure, add 160ml of ethyl acetate and 320ml of n-hexane to the residue and stir for 30 minutes, lower the temperature below 10°C, and start suction filtration to obtain a white solid , controlled temperature at 65°C, dried to obtain 104.8g of 2',3'-di-O-acetyl-5'-deoxy-5-fluoro-N4-n-pentyloxycarbonylcytidine, yield 91.6%, purity 99.7% .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com