Pomalyst gastric-soluble pellet and preparing method thereof
A technology of malidomide pellets and pomalidomide, applied in the field of pharmacy, to achieve the effects of improving drug stability, easy storage, and accurate dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Embodiment 1 preparation of pomalidomide stomach-dissolving type pellets
[0053] 1.1 Prescription
[0054] A. Pomalidomide gastric-soluble pellets
[0055] pomalidomide
2g
Starch core
150g
10g
talcum powder
3g
Gastric-soluble coating powder
10g
300g
100g
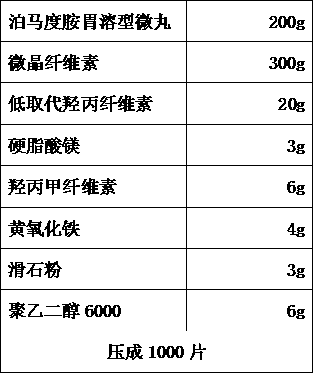
[0056] B. Pomalidomide stomach-soluble pellets
[0057]
[0058] 1.2 The method for preparing the above-mentioned prescription pomalidomide gastric-dissolved pellets is carried out according to the following steps:
[0059] 1) adding hypromellose to water, heating to make it completely dissolve, cooling, adding pomalidomide, after it is completely dissolved, dispersing talcum powder evenly in the solution;
[0060] 2) Place the starch pellet core in a fluidized bed, and then evenly spray the solution in "1)" on the surface of the starch pellet core with a fluidized bed device to make pomalidomide pelle...
Embodiment 2
[0067] Embodiment 2 preparation of pomalidomide stomach-dissolving type pellets
[0068] 2.1 Prescription
[0069] A. Pomalidomide gastric-soluble pellets
[0070] pomalidomide
1g
Starch core
100g
8g
talcum powder
3g
Gastric-soluble coating powder
10g
250g
100g
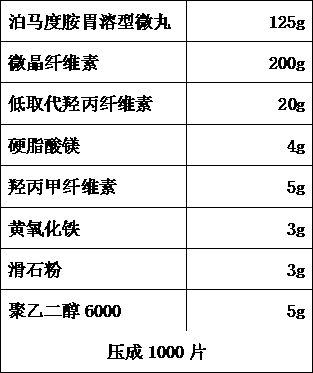
[0071] B. Pomalidomide stomach-soluble pellets
[0072]
[0073] 2.2 The method for preparing the above-mentioned prescription pomalidomide gastric-dissolved pellets is carried out according to the following steps:
[0074] 1) adding hypromellose to water, heating to make it completely dissolve, cooling, adding pomalidomide, after it is completely dissolved, dispersing talcum powder evenly in the solution;
[0075] 2) Place the starch pellet core in a fluidized bed, and then evenly spray the solution in "1)" on the surface of the starch pellet core with a fluidized bed device to make pomalidomide pell...
Embodiment 3
[0082] Embodiment 3 preparation of pomalidomide stomach-dissolving type pellets
[0083] 3.1 Prescription
[0084] A. Pomalidomide gastric-soluble pellets
[0085] pomalidomide
3g
Starch core
100g
hypromellose
6g
talcum powder
3g
Gastric-soluble coating powder
10g
260g
150g
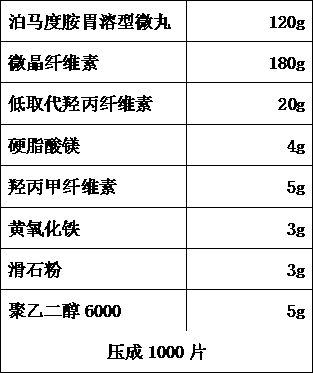
[0086] B. Pomalidomide stomach-soluble pellets
[0087]
[0088] 3.2 The method for preparing the above-mentioned prescription pomalidomide gastric-dissolved pellets is carried out according to the following steps:
[0089] 1) adding hypromellose to water, heating to make it completely dissolve, cooling, adding pomalidomide, after it is completely dissolved, dispersing talcum powder evenly in the solution;
[0090] 2) Place the starch pellet core in a fluidized bed, and then evenly spray the solution in "1)" on the surface of the starch pellet core with a fluidized bed device to make pomalidomide pell...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com