Diphenylaminopyridine type anti-tumor compound as well as preparation method and application thereof
A compound and anti-tumor technology, applied in the field of medicine, can solve the problems of limited application scope, toxic and side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The preparation of embodiment 1 target molecule
[0044] Use Yantai Huanghai GF254 or Qingdao GF254 silica gel plate for thin-layer chromatography. The specification of the silicon amine plate used in thin-layer chromatography (TLC) is 0.15mm-0.2mm, and the specification of thin-layer chromatography separation and purification products is 0.4 mm-0.5mm.
[0045] The raw materials used in the present invention are mainly purchased from Sinopharm Chemical Reagent Co., Ltd., Beijing Coup Technology Co., Ltd., Aladdin Chemical Reagent Co., Ltd., Darui Chemicals and other companies.
[0046] Unless otherwise specified in the examples, the solution refers to an aqueous solution.
[0047] Unless otherwise specified in the examples, the reaction temperature is room temperature, which is 20°C-30°C.
[0048] The technical scheme that the present invention adopts is as follows:
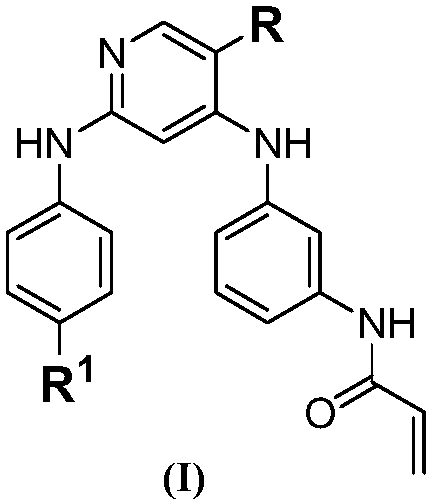
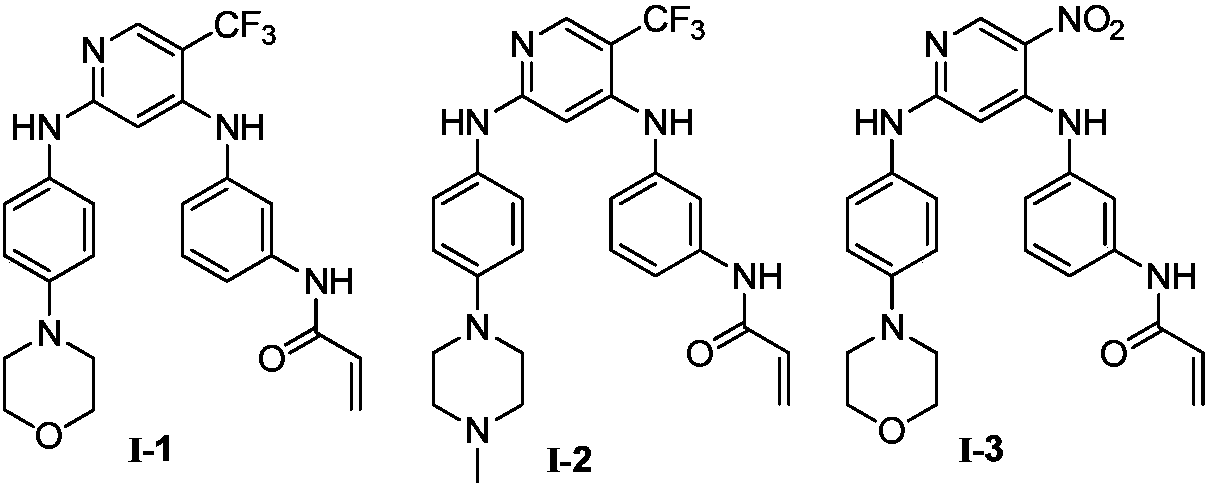
[0049]
[0050] Synthetic route of compound (I). Reagents and conditions: (a) m-nitroaniline, DIP...
Embodiment 2
[0078] Example 2 Evaluation of Target Molecule Biological Activity
[0079] 1. In vitro test method for receptor tyrosine kinase inhibitory activity
[0080] Prepare Kinase Assay Buffer
[0081] ① Melt the Kinase Detection Buffer at room temperature and observe whether there is precipitation.
[0082] ②If precipitation occurs, incubate Kinase Detection Buffer at 37°C for 15 minutes and shake frequently to dissolve the precipitation. Alternatively, carefully aspirate the supernatant to remove the pellet.
[0083] Prepare Kinase Assay Reagents
[0084] ①Equilibrate Kinase Detection Buffer and Kinase Detection Substrate at room temperature before use.
[0085] ② Pour all the Kinase Detection Buffer into the brown bottle containing the Kinase Detection Substrate to dissolve the lyophilized powder substrate, thus making the Kinase Detection Reagent.
[0086] ③Gently oscillate, vortex or invert to mix to form a homogeneous solution, and the substrate should be dissolved within ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com