A 13-valent pneumococcal polysaccharide-protein conjugate composition and its preparation method and application
A pneumococcal polysaccharide and pneumococcal technology, applied in the field of 13-valent pneumococcal polysaccharide-protein conjugate composition and its preparation, can solve the problem of affecting the immunogenicity and safety of pneumococcal polysaccharide conjugates and affecting the free protein content of carrier proteins And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] The preparation of embodiment 1 tetanus toxoid carrier protein
[0059] Clostridium tetani with the strain number CMCC 64008 was obtained from the Medical Bacteria Preservation Management Center of the China Institute for the Control of Biological Products. The original seed bank, main generation seed bank and working seed bank were established with the above strains. The original seed batch is the 0th generation, the second generation from the original seed batch is the main generation seed batch, and the second generation from the main generation seed batch is the working seed batch. Start the batch of working seed strains, inoculate in semi-solid medium, 35°C, 48-hour expansion culture, pass three generations to make production seeds. Prepare the tetanus toxin-producing medium according to the formula in Table 1, and use it after high-pressure steam sterilization.
[0060] Table 1 Tetanus toxigenic medium formula
[0061] serial number Material name Q...
Embodiment 2
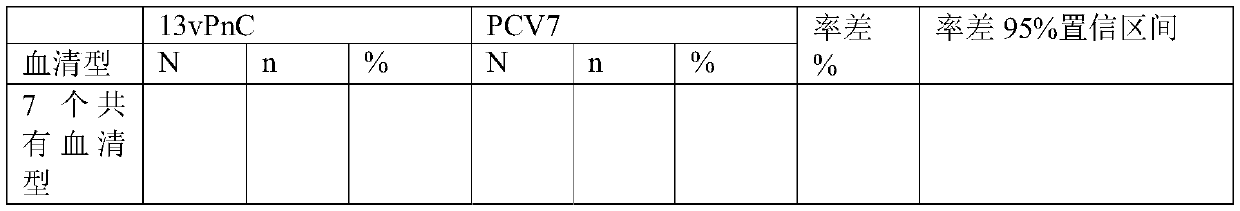
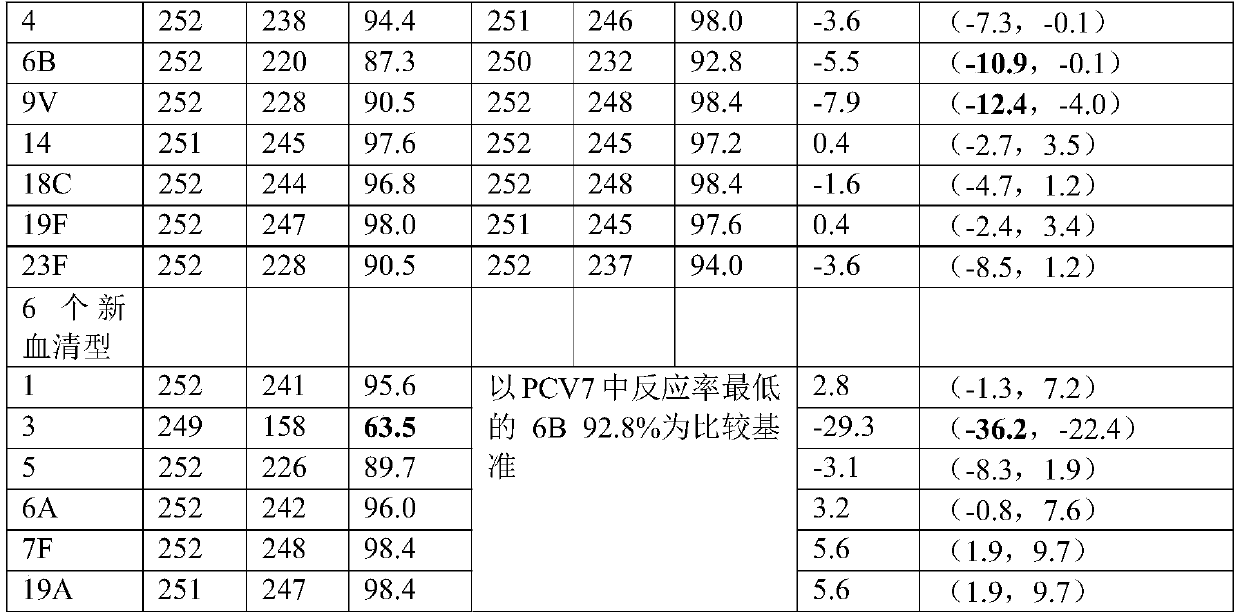
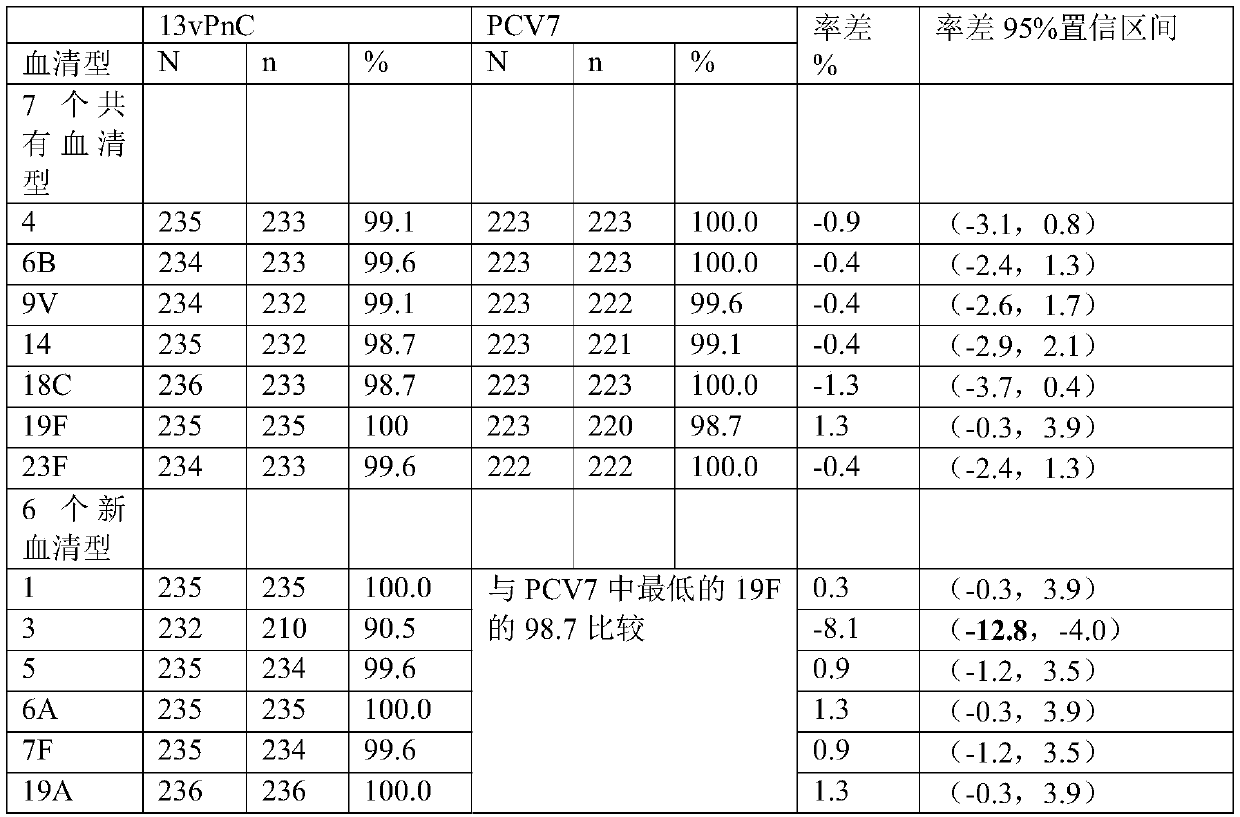
[0069] Example 2 Degradation of pneumococcal type 1, type 3, type 4, type 5, type 6B, type 7F, type 18C, type 19A polysaccharide
[0070] Pneumococcal type 1, type 3, type 4, type 5, type 6B, type 7F, type 18C, and type 19A polysaccharides were dissolved in 0.2mol / L NaCl to 2-8mg / ml respectively, and prepared as solutions; Pre-cooling in ice bath conditions; ultrasonic treatment in ice bath conditions, ultrasonic power of 350-400W, ultrasonic for 3 seconds, pause for 5 seconds, ultrasonic for 2-20 minutes. Various types of ultrasonic conditions are shown in Table 3.
[0071] Table 3 polysaccharide ultrasonic process parameters
[0072] Ultrasound time type 1 polysaccharide 5 type 3 polysaccharide 10 type 4 polysaccharide 8 type 5 polysaccharide 2 Type 6B polysaccharide 6 7F polysaccharide 15 18C polysaccharide 20 19A polysaccharide 12
[0073] Determination of main peak K of polysaccharides after ultrasonic degr...
Embodiment 3
[0077] Example 3 Degradation of pneumococcal 6A type, 9V type, 14 type, 19F type, 23F type polysaccharide
[0078] Pneumococcal type 6A, 9V, 14, 19F, and 23F polysaccharides were dissolved in 0.2mol / L NaCl to 2-8mg / ml respectively, and prepared as a solution; they were placed in constant temperature equipment, and the temperature was controlled at 70°C- 85°C, time 0.5-2h, take out and cool naturally. The hydrolysis conditions of each type are shown in Table 5.
[0079] Table 5 polysaccharide hydrolysis process parameters
[0080] Control temperature(℃) Hydrolysis time (h) Type 6A polysaccharide 70 1.5 9V polysaccharide 80 1.0 Type 14 polysaccharide 85 0.5 19F polysaccharide 75 1.0 23F polysaccharide 70 2
[0081] Determination of the main peak K of polysaccharides degraded by high temperature using Sepharose CL-4B XK16 / 100 column D Value, the mobile phase is 0.9% sodium chloride, the flow rate is 0.3ml / min, and 4.5ml / 15mi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com