Phenylacrylate compound as well as preparation method and application thereof
A technology of phenylacrylate and compound, applied in the field of phenylacrylate compound and preparation thereof, can solve the problems such as not being able to show ideal medicinal effect and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Synthesis of Compound A: Under nitrogen protection, malonic acid mono(1R,2S,4R)-1,7,7-trimethylbicyclo[2.2.1]heptane-2-ol ester (11.5g, 47.9 mmol), 3-fluoro-4-hydroxyl-5-methoxybenzaldehyde (7.3g, 42.9mmol) were added in toluene (100ml), added pyridine (7.6g, 95.8mmol), piperidine (0.4g, 4.7 mmol), the temperature was raised to 120°C for 5 hours. After the reaction was completed, the reaction solution was allowed to cool, and 1N hydrochloric acid solution, saturated NaHCO 3 solution, washed with saturated NaCl solution, dried over anhydrous sodium sulfate for 4 h, concentrated to dryness, and recrystallized from ethyl acetate / petroleum ether to obtain 10.2 g of white powder solid with a yield of 68.5%. EI-MS M / Z349.2[M + ], 347.3[M - ].
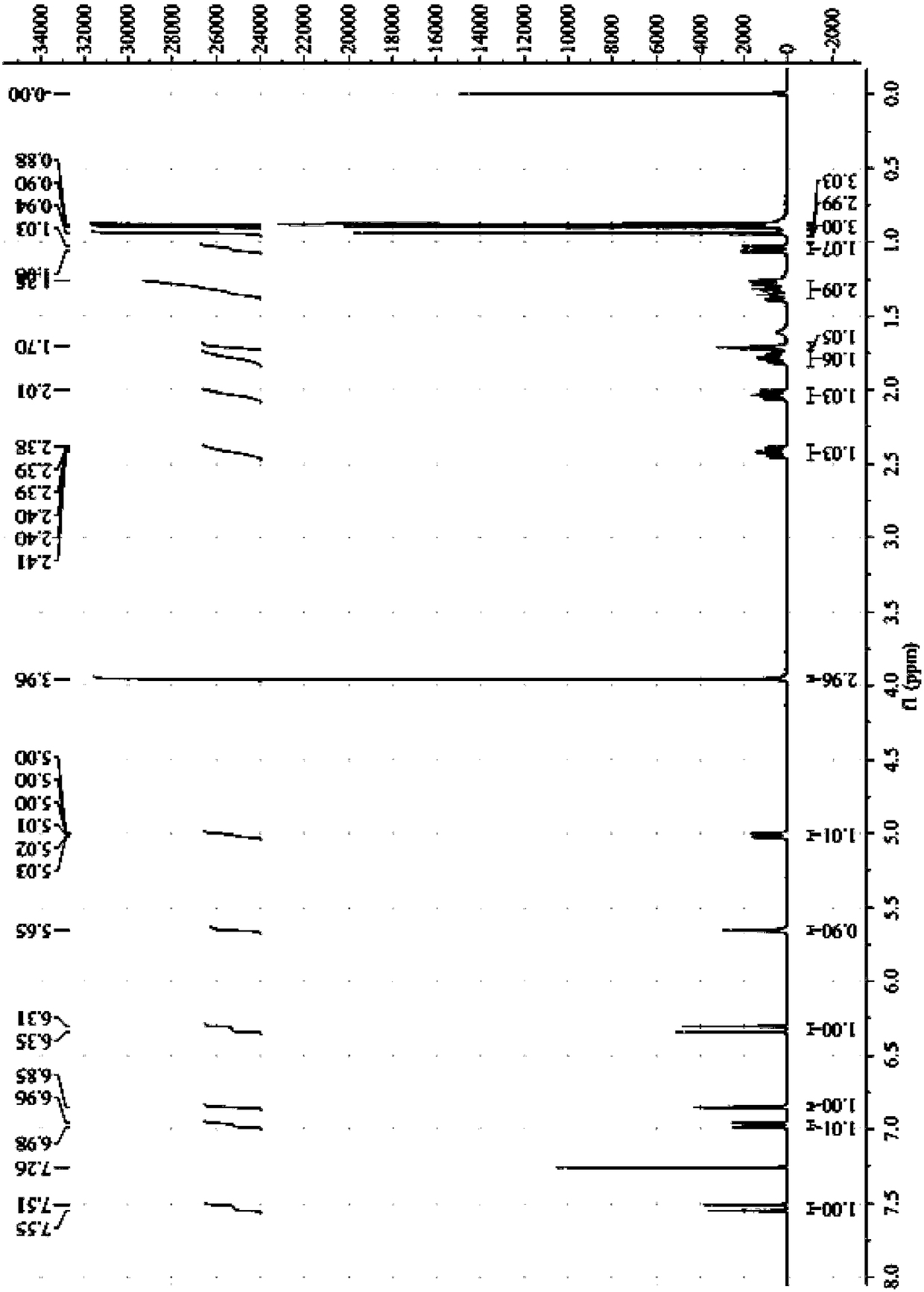
[0088] 1 H-NMR (CDCl 3 ,500MHz) 0.88, 0.90, 0.94 (each 3H, s, H-8, 9, 10), 1.05 (1H, dd, J=17.2, 4.3Hz, H-3b), 1.25 (2H, m, H-5b , H-6b), 1.70(1H,m,H-4),1.78(1H,m,H-5a),2.01(1H,m,H-6a),2.41(1H,m,H-3a), 3.96 (3H, s, -OMe), 5.01 (...
Embodiment 2
[0090] Synthesis of Compound A: Under nitrogen protection, malonic acid mono(1R,2S,4R)-1,7,7-trimethylbicyclo[2.2.1]heptane-2-ol ester (5.3g, 22.1 mmol), 3-fluoro-4-hydroxyl-5-methoxybenzaldehyde (3.0g, 17.6mmol) were added in toluene (50ml), piperidine (0.38g, 4.4mmol), acetic acid (0.26g, 4.4 mmol), the temperature was raised to 120°C for 5 hours. After the reaction was completed, the reaction solution was allowed to cool, and 1N hydrochloric acid solution, saturated NaHCO 3 solution, washed with saturated NaCl solution, dried over anhydrous sodium sulfate for 4 h, concentrated to dryness, and recrystallized from ethyl acetate / petroleum ether to obtain 4.3 g of white powder solid, with a yield of 70.5%.
Embodiment 3
[0092] Synthesis of Compound B: Under nitrogen protection, malonic acid mono(1R,2S,4R)-1,7,7-trimethylbicyclo[2.2.1]heptane-2-ol ester (11.5g, 47.9 mmol), 3,4-dihydroxy-5-methoxybenzaldehyde (7.2g, 42.9mmol) were added to toluene (100ml), and pyridine (7.6g, 95.8mmol), piperidine (0.4g, 4.7mmol) were added ), heated up to 120°C for 5 hours, after the reaction was over, let the reaction liquid cool down, and washed with 1N hydrochloric acid solution, saturated NaHCO 3 solution and saturated NaCl solution, dried over anhydrous sodium sulfate for 4 h, concentrated to dryness, and recrystallized from ethyl acetate / petroleum ether to obtain 8.3 g of white powder solid, with a yield of 55.8%. EI-MS M / Z347.2[M + ], 345.2[M - ].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com