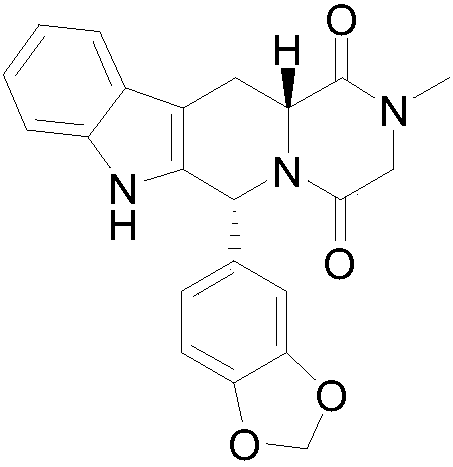
Refining method of tadalafil crystal form I
A technology of tadalafil and purification method, applied in the field of medicinal chemistry, can solve the problems of large amount of solvent, low yield and high cost, and achieve the effects of improving quality and yield, simple preparation method and high reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
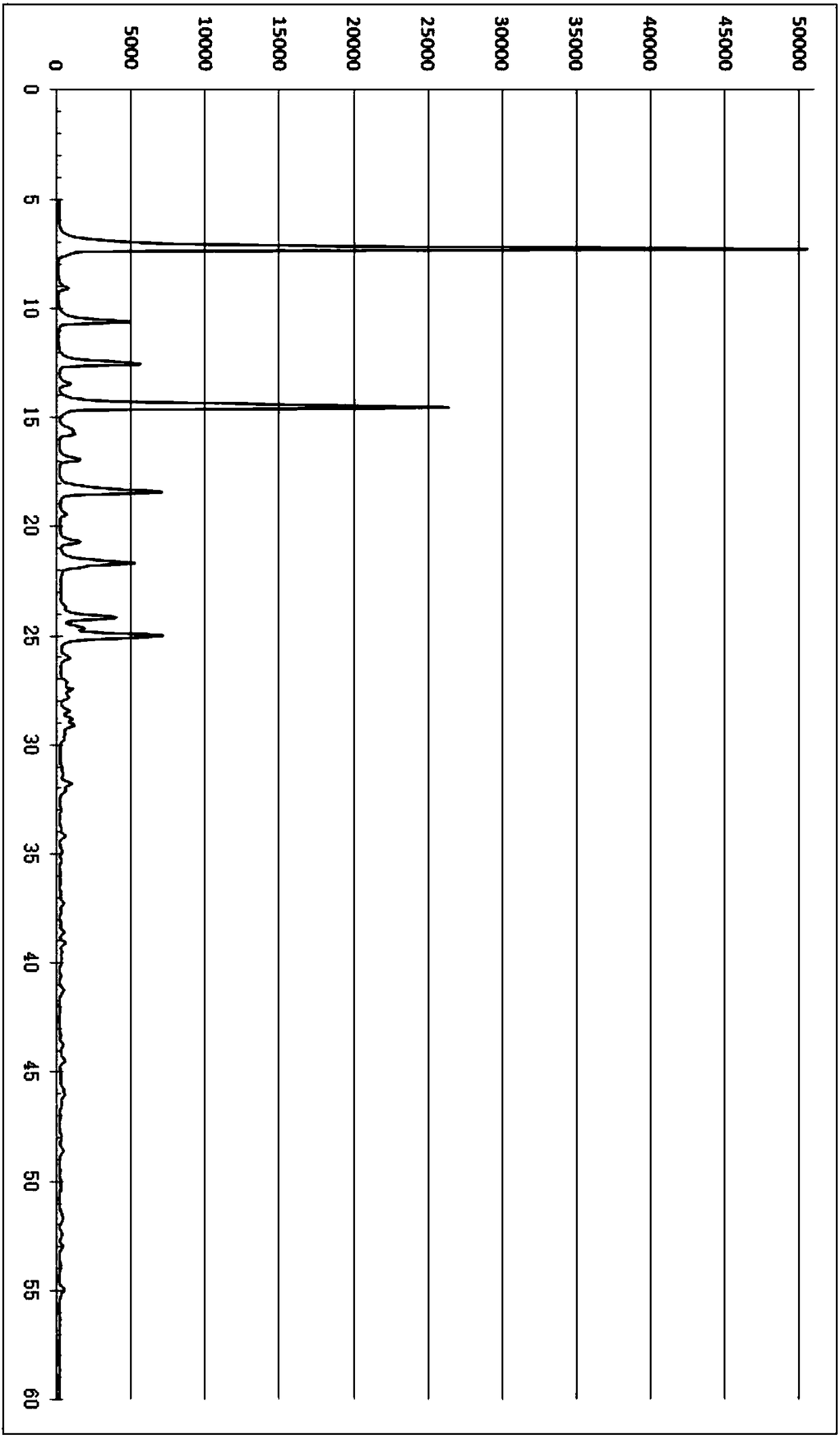
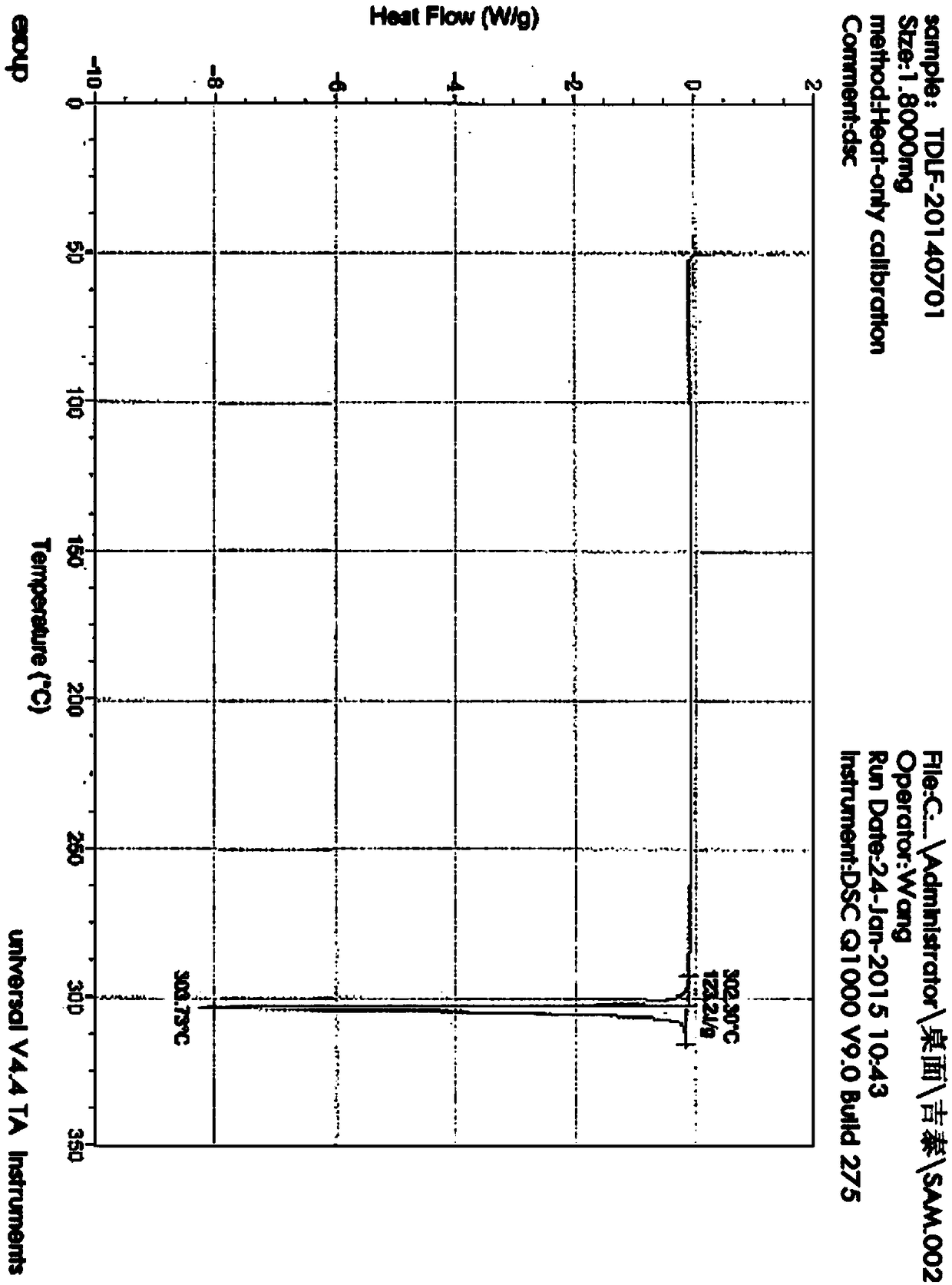
[0038] Add 10.00 g of crude tadalafil to a 500 mL three-neck round bottom flask, and then add 150 g of acetic acid / methanol / water mixed solvent (acetic acid:methanol:water mass ratio 95:5:1). Start stirring, raise the temperature to 90-110°C, dissolve the material, cool down to 80-90°C, add activated carbon and stir to raise the temperature to 90-110°C, then keep warm and stir for 30 minutes. After filtering, the temperature of the filtrate was lowered to 30-40°C, crystals were precipitated, and 200 g of water was slowly added. Cool down to 0-5°C and continue crystallization for 60 minutes, filter, wash with water, and dry in vacuum at 75-85°C for 8 hours to obtain 9.68g of Tadala amorphous form A, yield 96.8%, purity (HPLC): 99.83% , Impurity A (HPLC): 0.07%, Impurity 2 (HPLC): 0.06%.
Embodiment 2
[0040] Add 10.00 g of crude tadalafil to a 500 mL three-necked round bottom flask, and then add 150 g of acetic acid / methanol / water mixed solvent (acetic acid:methanol:water mass ratio 99:10:6). Start stirring, raise the temperature to 90-110°C, dissolve the material, cool down to 80-90°C, add activated carbon and stir to raise the temperature to 90-110°C, then keep warm and stir for 30 minutes. After filtering, the temperature of the filtrate was lowered to 30-40°C, crystals were precipitated, and 200 g of water was slowly added. Cool down to 0-5°C and continue crystallization for 60 minutes, filter, wash with water, and dry in vacuum at 75-85°C for 8 hours to obtain Tadalamorph A9.63g, yield 96.3%, purity (HPLC): 99.99% , Impurity A (HPLC): N.D, Impurity 2 (HPLC): N.D.
Embodiment 3
[0042] Add 10.00 g of crude tadalafil to a 500 mL three-neck round bottom flask, and then add 150 g of acetic acid / methanol / water mixed solvent (acetic acid:methanol:water mass ratio 96:7:3). Start stirring, raise the temperature to 90-110°C, dissolve the material, cool down to 80-90°C, add activated carbon and stir to raise the temperature to 90-110°C, then keep warm and stir for 30 minutes. After filtering, the temperature of the filtrate was lowered to 30-40°C, crystals were precipitated, and 200 g of water was slowly added. Cool down to 0-5°C and continue crystallization for 60 minutes, filter, wash with water, and dry in vacuum at 75-85°C for 8 hours to obtain Tadalamorph A9.72g, yield 97.2%, purity (HPLC): 99.96% , Impurity A (HPLC): 0.02%, Impurity 2 (HPLC): N.D.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com