Production and purification process of ethyl lauroyl arginate hydrochloride
A technology of lauroyl arginine ethyl ester hydrochloride and lauroyl arginine ethyl ester, which is applied in the production field of lauroyl arginine ethyl ester hydrochloride, can solve problems such as incomplete repeatable realization, and avoid The effect of the hydrolysis problem
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] This example is implemented according to the technical solution disclosed in Example 2 of the patent WO 2013 / 098659 A1, as a comparative example of the patent of the present invention.
[0043] Weigh 27.5kg (100mol) of L-arginine ethyl ester dihydrochloride and add it into 100L tetrahydrofuran and stir until it dissolves completely, then add 20.2kg of triethylamine (200mol) slowly into the system and stir for 30min. 22 kg of lauroyl chloride (100 mol) was added dropwise under the conditions, and the reaction was continued for 2 hours. After adding 10.1 kg of triethylamine (100 mol), the temperature was raised to 40° C. until the reaction was complete. Remove triethylamine hydrochloride by filtration, concentrate the dry system to obtain the crude product, add 250L of water, cool the system to below 10°C and adjust the pH to acidic with 10% dilute hydrochloric acid. Add 250L of ethyl acetate, heat up to 30°C to dissolve the extracted product as much as possible, add 25L ...
Embodiment 2
[0045] The production and purification process of a kind of lauroyl arginine ethyl ester hydrochloride provided by the present invention adopts the following steps to prepare and purify lauroyl arginine ethyl ester hydrochloride:
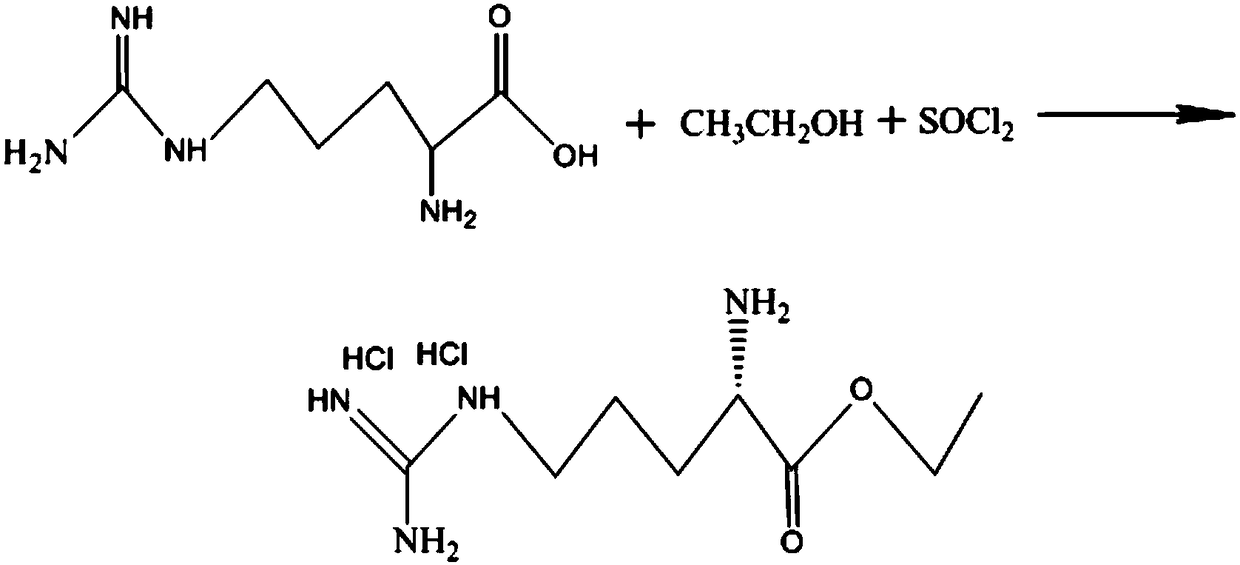
[0046] Step A: Add 10.5kg (60.3mol) of L-arginine and 95kg of ethanol to a 200L reactor at room temperature, and slowly add 26kg (218.48mol) of thionyl chloride dropwise. Within 25°C, after the dropwise addition, the system was dissolved and clarified, and the reaction was completed at 45°C for 10 hours. Concentrate dry ethanol to obtain ethyl arginine dihydrochloride, and proceed to the next step of reaction.
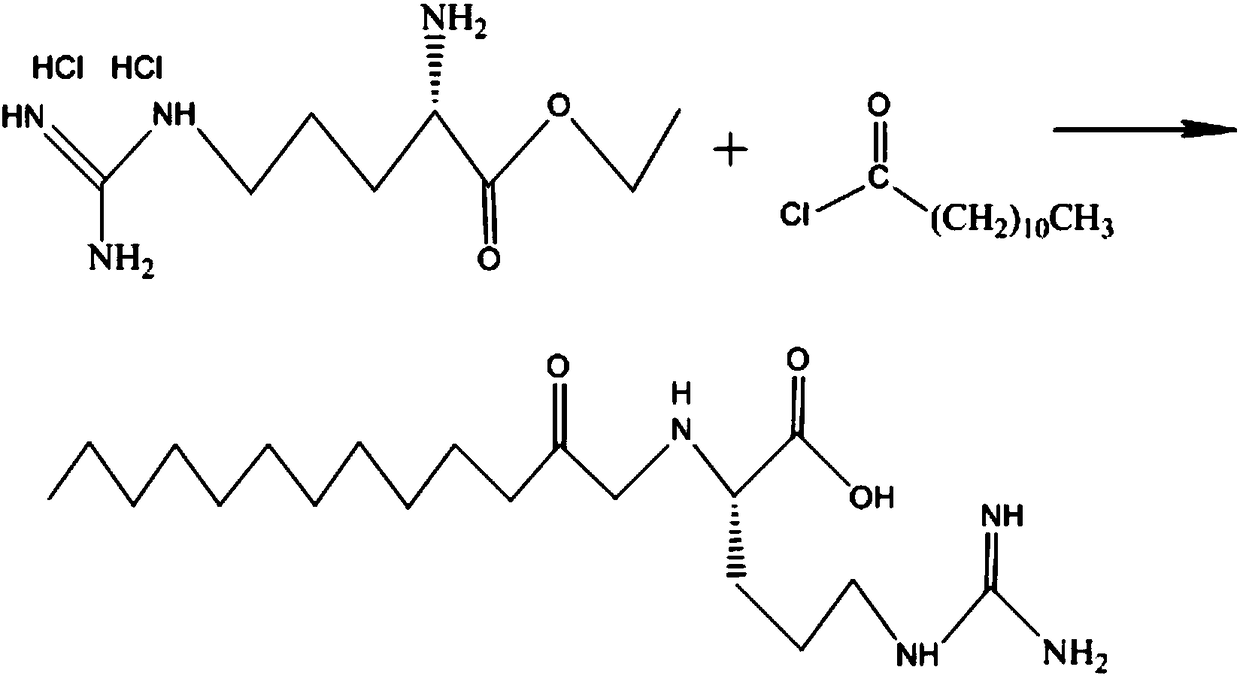
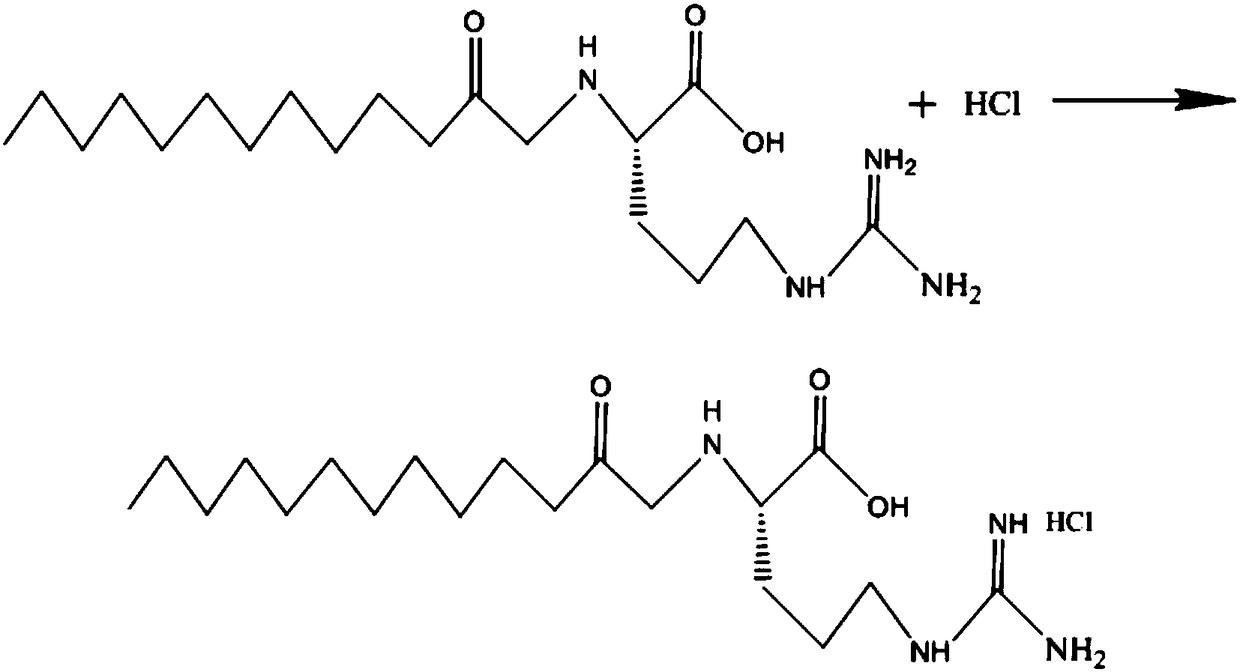
[0047] Step B: Add 120 kg of chloroform to the reaction product of the previous step, cool down to 5°C in the kettle, add 12 kg (118.8 mol) of triethylamine dropwise, stir for 1 h until the system is dissolved and clear, then add 13.45 kg (61.48 mol) of lauroyl chloride dropwise ), the reaction is exothermic, and the temperature is controlle...
Embodiment 3
[0051] This embodiment further carries out process enlargement on the basis of embodiment 2, and its production purification process adopts the following steps to prepare and purify lauroyl arginine ethyl ester hydrochloride:
[0052] Step A: Add 50kg (287.35mol) of L-arginine and 450kg of ethanol to a 1000L reaction kettle at room temperature, slowly add 130kg (1092.43mol) of thionyl chloride dropwise, the dropping process releases heat, and the dropping temperature is controlled to 25°C Within a period of time, after the dropwise addition, the system was dissolved and clarified, and the reaction was completed at 30°C for 18 hours. Concentrate dry ethanol to obtain ethyl arginine dihydrochloride, and proceed to the next step of reaction.
[0053] Step B: Add 750 kg of dichloromethane to the reaction product of the previous step, lower the temperature in the kettle to 5°C, add 60 kg (594 mol) of triethylamine dropwise, stir for 1 h until the system is dissolved and clear, then ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com