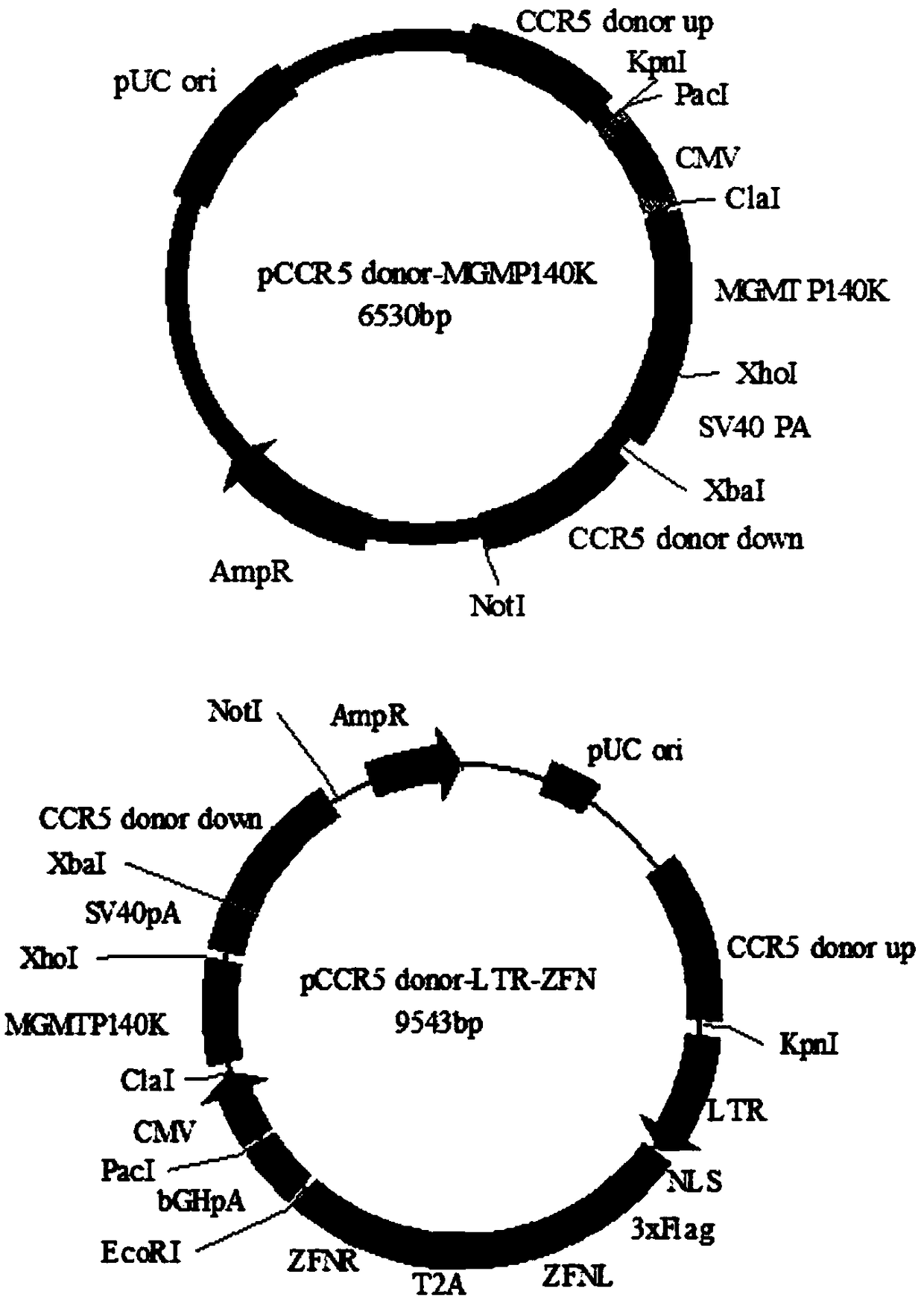
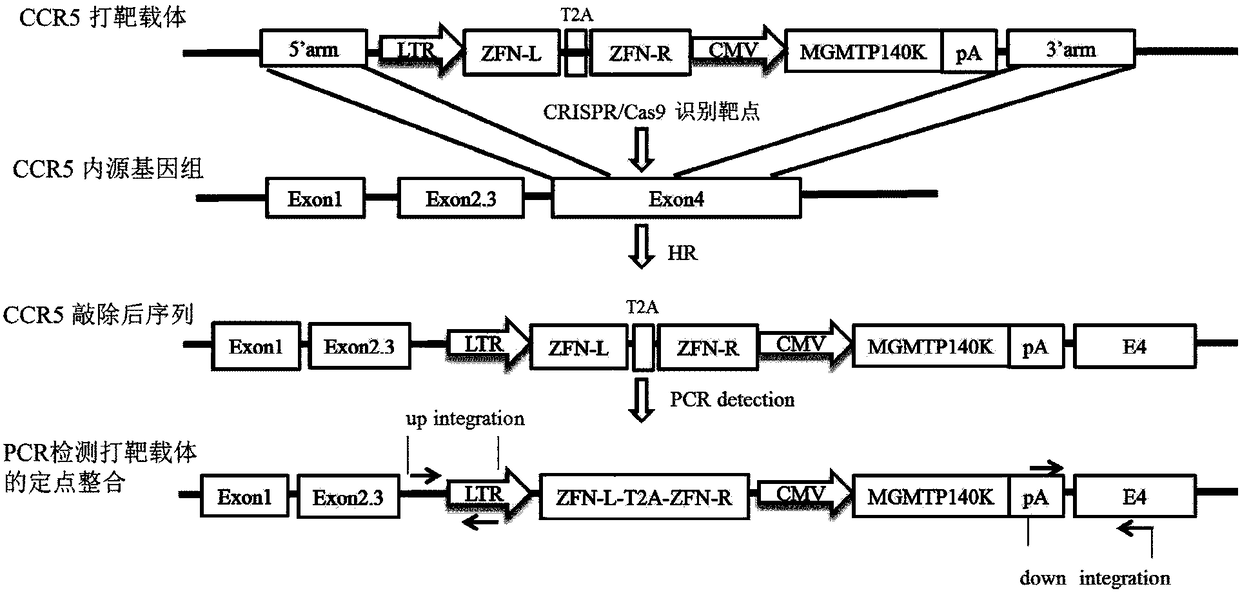
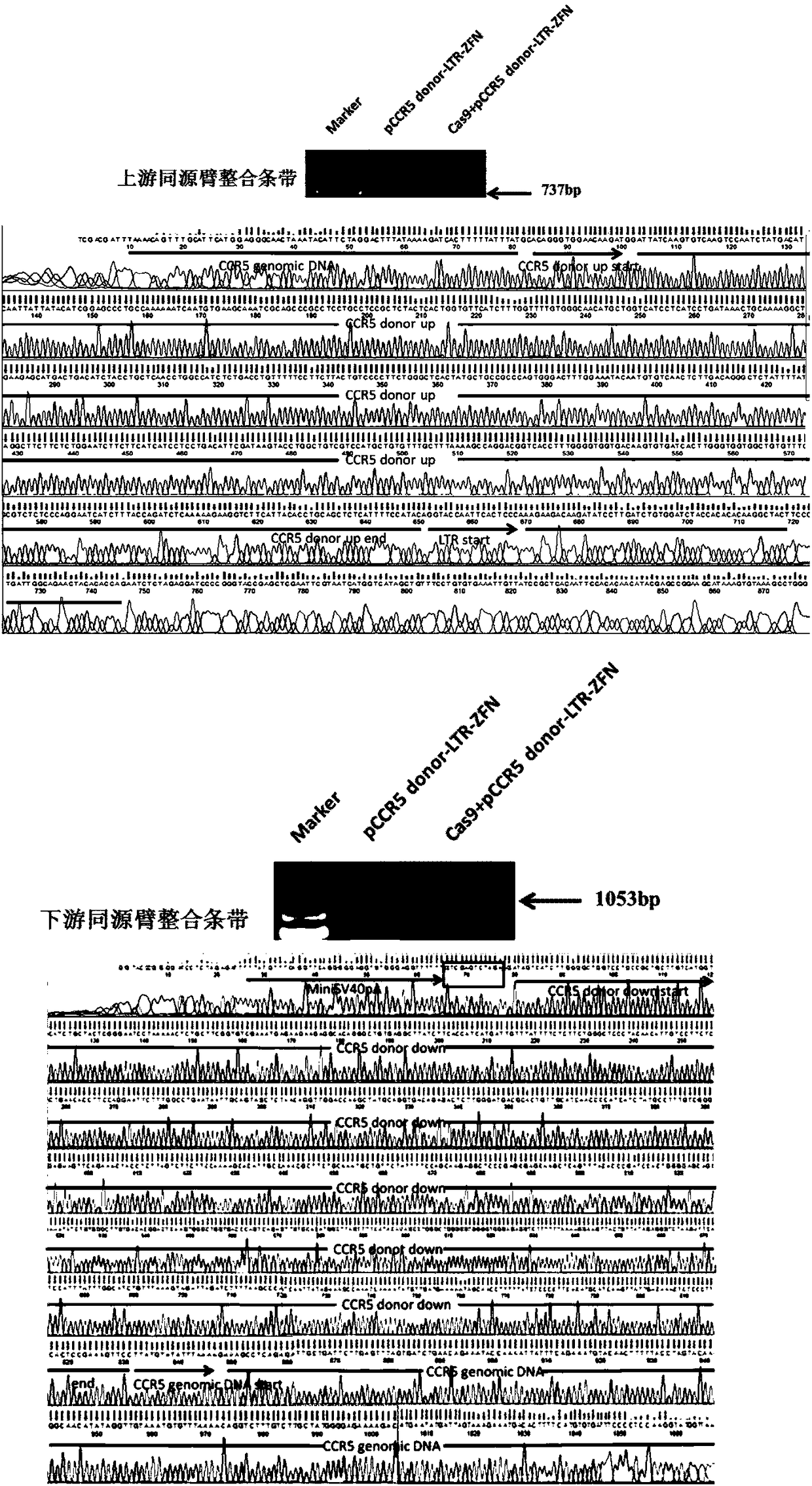
Anti-HIV/AIDS gene targeting vector, construction method and application
A gene targeting and carrier technology, applied in the direction of carrier, nucleic acid carrier, genetic engineering, etc., can solve problems such as side effects, genotoxicity, and antiviral drugs that cannot eradicate viruses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Gene recombination detection of gene targeting vector
[0032] Human embryonic kidney cells HEK293 were used as target cells, and the gene editing vector CRISPR / Cas9 and the gene targeting vector pCCR5 donor-LTR-ZFN (the mass ratio of the two were 1:5) were co-transfected into HEK293 cells by transient transfection , pcDNA3.1(-) and gene targeting vector pCCR5 donor-LTR-ZFN co-transfection group was used as the control group; on the fifth day after transfection, the cells were screened with drugs, and the medium was changed to cultivate drug-resistant cells. When the cells no longer appear to die, stop the drug screening; extract the cell genome, and use specific primers to amplify the integration of the gene targeting vector at the CCR5 site to confirm that these surviving drug-resistant cells are genetically modified positive cells, agar The results of sugar gel electrophoresis showed that in the cells co-transfected with CRISPR / Cas9 and the gene targeting v...
Embodiment 2
[0034] The biological activity detection of embodiment 2 antiviral element
[0035] The genetically modified cells obtained above were inoculated into 24-well plates, and then the HIV-1 virus plasmid pHIV-NL4-3-luc carrying the luciferase reporter gene luciferase and the internal reference gene expression plasmid pRL-SV40pA were transfected into the above two In this cell line, after three days, the cells were harvested and lysed, and the resulting supernatant was used for the detection of luciferase activity; the results of the analysis of the dual luciferase reporter detection system showed that it was different from the control cell line that only integrated the screening marker gene ( MGMTP140K), in the cell line integrated with ZFN antiviral elements, the expression of luciferase was nearly 30% decreased (eg Figure 4 shown);
[0036] In order to further verify the antiviral activity of ZFN, the above two genetically modified cell lines were infected with the pseudotyped ...
Embodiment 3
[0037] Detection of CCR5 molecular expression in embodiment 3TZM-bl cells
[0038]Taking TZM-bl cells expressing CCR5 and CXCR4 molecules as target cells, the gene editing vector CRISPR / Cas9 and the gene targeting vector pCCR5 donor-MGMTP140K or pCCR5 donor-LTR-ZFN were co-introduced into TZM-bl by transient transfection, On the fifth day, the BCNU / BG dual drug was used for screening. When the cells no longer died, the obtained cells were drug-resistant cells; then expanded culture, and finally, the expression of CCR5 in the above-mentioned drug-resistant cells was detected by flow cytometry The change of; by flow cytometry detection and analysis, the results showed that, compared with the untreated group (Mock), the expression level of CCR5 in TZM-bl cells (named MGMTP140K and ZFN respectively) transfected with gene editing vector and gene targeting vector Significant decrease (such as Figure 5 shown).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com