Perfluorocarbon albumin nanoparticles and preparation method and application thereof
An albumin nanoparticle and perfluorocarbon technology, which is applied in the field of preparation of albumin nanoparticles, can solve the problems of high requirements, influence drug safety, and limit the application of preparations related to albumin nanoparticles, and achieves a simple and easy preparation process. , the effect of improving biological safety and low equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Preparation of perfluorotributylamine (PFTBA)-albumin nanoparticles:
[0042] Measure 0.5mL of human serum albumin stock solution (10g / 50mL) into a 7mL vial, add deionized water to dilute to 2mL, and mix well at 20°C. Then add 1.5mL perfluorotributylamine (PFTBA), then add 1.0mL ethanol (analytical grade), and mix well. Use an ultrasonic probe to go deep into the vial to the bottom of the liquid, use 500W power during ultrasonication, set the ultrasound to stop for 3s after every 2s, ultrasonication 3 times, each time 2 minutes, and the preparation needs to be placed in a 20°C water bath after each ultrasonication 1-2 minutes. Transfer the sample to a 4mL centrifuge tube, centrifuge at 3000rpm for 3min, and take the supernatant, which is the prepared perfluorotributylamine albumin nanoparticles.
[0043]The morphology of perfluorotributylamine-albumin nanoparticles was observed by transmission electron microscope (TEM). First, the prepared preparation was diluted 160...
Embodiment 2
[0045] Preparation of perfluorooctyl bromide (PFOB)-albumin nanoparticles:
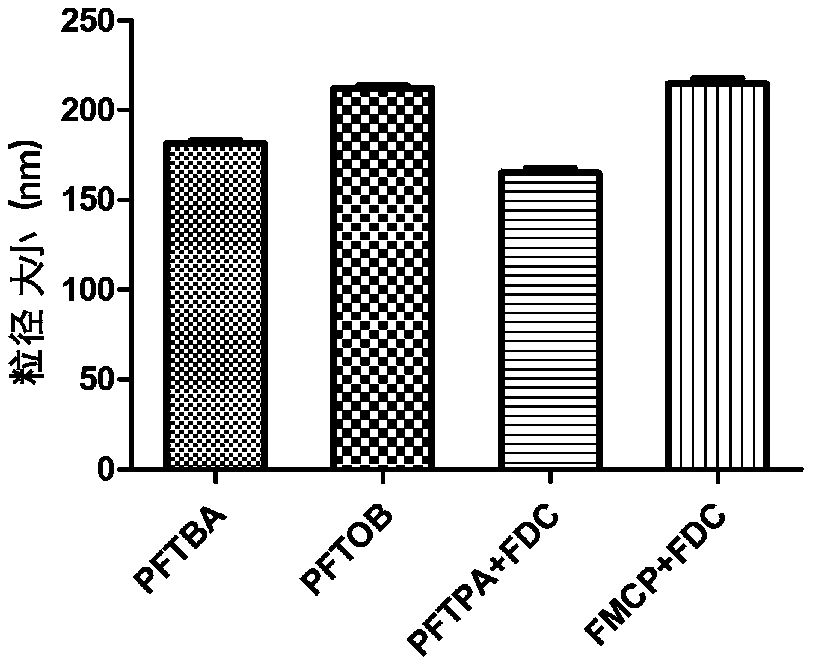
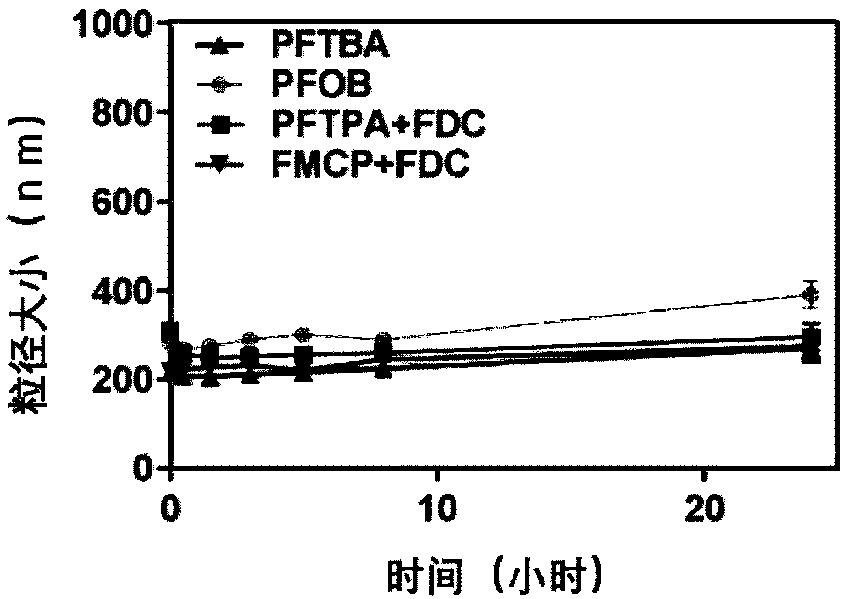
[0046] Measure human serum albumin stock solution and add water to dilute to albumin concentration of 5g / 100mL, and mix well at 25°C. Take 2 mL of the above-mentioned diluted solution, add 3 g of perfluorooctyl bromide (PFOB), and then add 1.0 mL of ethanol (analytical grade), and mix well. Use an ultrasonic probe to penetrate deep into the bottle to near the bottom of the liquid for sonication. Transfer the sample to a centrifuge tube, centrifuge at 3000rpm for 3min, and take the supernatant, which is the prepared perfluorooctyl bromide albumin nanoparticles with an average particle size of 220nm.
Embodiment 3
[0048] Preparation of perfluorotripropylamine (PFTPA) + perfluorodecalin (FDC)-albumin nanoparticles:
[0049] Measure human serum albumin stock solution and add water to dilute to albumin concentration of 5g / 100mL, and mix well at 30°C. Take 2 mL of the above diluted solution, add 0.33 g of perfluorotripropylamine and 0.79 g of perfluorodecalin, then add 1.0 mL of ethanol (analytical grade), and mix well. Use an ultrasonic probe to penetrate deep into the bottle to near the bottom of the liquid for sonication. Transfer the sample to a centrifuge tube, centrifuge at 3000rpm for 3min, and take the supernatant, which is the prepared perfluorotripropylamine perfluorodecalin albumin nanoparticles, with an average particle size of 150nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com