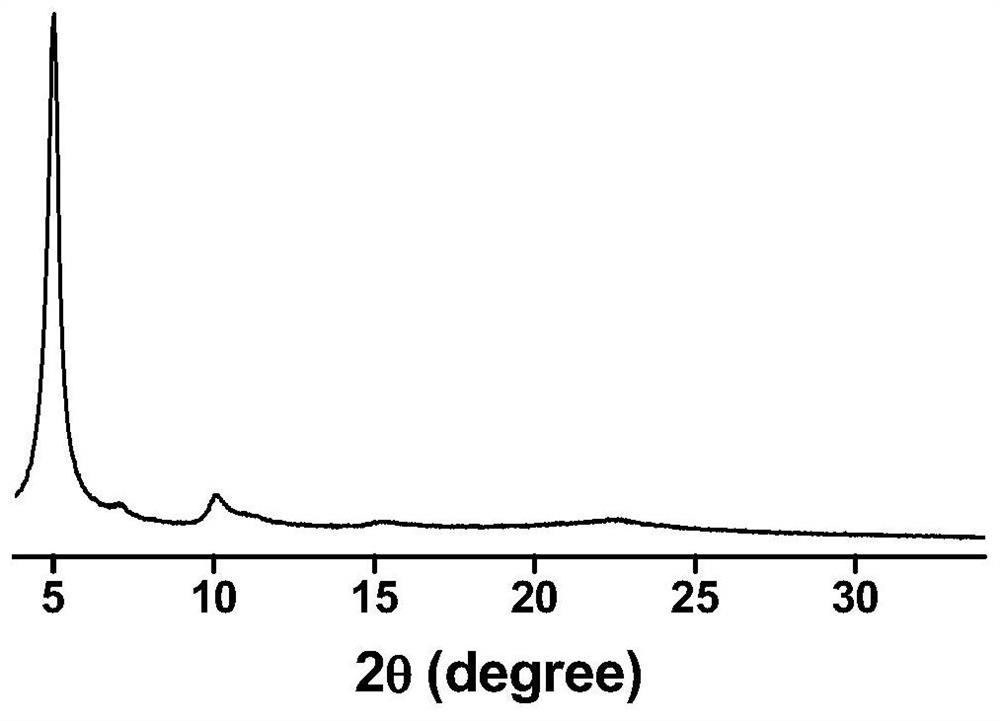
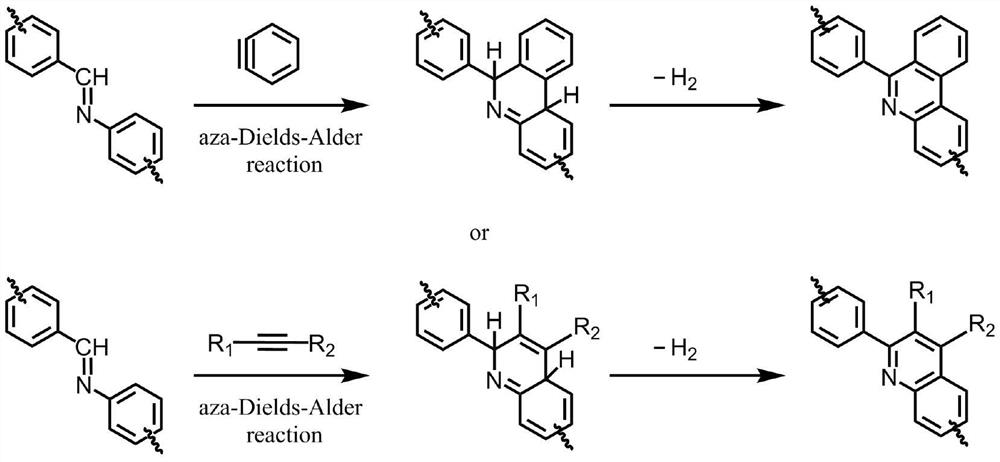
A method to improve the chemical stability of organic framework porous materials
A chemically stable and porous material technology, applied in the field of improving the chemical stability of organic framework porous materials, can solve problems such as COFs destruction, achieve the effects of improving acid resistance, wide application range, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] A method for improving the chemical stability of an organic framework porous material, the steps are as follows:
[0023] (1) In dry 1,2-dichloroethane solvent, add 50 mg of freshly prepared TF-Py COF material, and obtain a suspension of TF-Py COF by ultrasonic dispersion.
[0024] (2) Slowly add 0.052 g of o-carboxylic acid benzene diazonium into the suspension according to the molar ratio of its imine bond to TF-Py COF is 2:1.
[0025] (3) Under the condition of sufficient stirring, the temperature was raised to 100° C., the reaction was refluxed for 24 hours, and then the reaction was terminated.
[0026] (4) The reacted COFs powder was filtered, washed thoroughly with a large amount of anhydrous tetrahydrofuran and anhydrous acetone, and dried under vacuum at 80°C to obtain the modified TF-Py COF material. After standing the TF-Py COF before modification in the hydrochloric acid solution with pH = 1 or the sodium hydroxide solution with pH = 14 for 30 min, it was f...
Embodiment 2
[0028] A method for improving the chemical stability of an organic framework porous material. The reaction conditions and preparation steps are the same as in Example 1, except that the molar ratio of the alkyne compound to the COF imine bond is 1:1. After the treated TF-Py COF was left standing in the hydrochloric acid solution of pH=1 or the sodium hydroxide solution of pH=14 for 24 hours, the TF-Py COF still had a good crystal form.
Embodiment 3
[0030] A method for improving the chemical stability of organic framework porous materials, the reaction conditions and preparation steps are the same as those in Example 1, except that the reaction temperature is 140°C. After the treated TF-Py COF was left standing in the hydrochloric acid solution of pH=1 or the sodium hydroxide solution of pH=14 for 24 hours, the TF-Py COF still had a good crystal form.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com