A New Contrast Agent for CT
A contrast agent, CH2 technology, applied in the preparation of X-ray contrast agents, preparations for in vivo tests, preparation of organic compounds, etc., can solve problems such as increased viscosity of the system, adverse health of patients, and enhanced osmotic pressure, and achieve resolution The effect of high efficiency, clear and visible images, and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
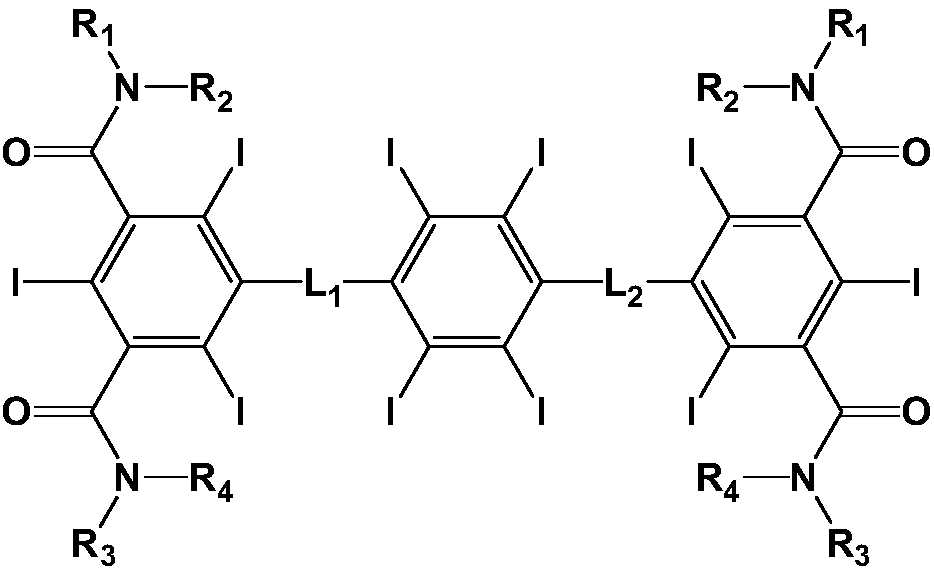
[0043] Example 1: Decaiodool-101
[0044]
[0045] Add 10ml of thionyl chloride into the reaction flask, lower the temperature to 10°C, then add 3.2g of (tetraiodo-1,4-phenylene)dimethanol, heat up to 50°C and stir for 2 hours after the addition, and distill off after the reaction Add 100ml of ethanol to dissolve thionyl chloride for later use.
[0046] 5-amino-N 1 -(2,3-Dihydroxypropyl)-N 3 -Hydroxymethyl-2,4,6-triiodo-N 1 Dissolve 7.41g of -methyl-isophthalamide in 200ml of ethanol, add 10ml of saturated sodium hydroxide solution, add the solution obtained in the previous step dropwise under stirring, raise the temperature to reflux after the dropwise addition, continue stirring for 2h, and filter while hot. The filtrate was rotary evaporated to remove the solvent, adjusted to pH 3-4 with 4N hydrochloric acid solution, stirred for 0.5 h, left to stand for crystallization for 3 h, then recrystallized from ethanol, filtered, and the filter cake was dried under vacuum to ...
Embodiment 2
[0049] Example 2: Decaiodool-102
[0050]
[0051] According to the method of Example 1, replace (tetraiodo-1,4-phenylene) dimethanol with bis(4-hydroxybutyl) 2,3,5,6-tetraiodoterephthalate, and use 5 -Amino-N 1 -(2,3-Dihydroxypropyl)-N 3 -Hydroxymethyl-2,4,6-triiodo-isophthalamide instead of 5-amino-N 1 -(2,3-Dihydroxypropyl)-N 3 -Hydroxymethyl-2,4,6-triiodo-N 1 -Methyl-isophthalamide, the target product was obtained with a yield of 67.2%.
[0052] ESI-MS: 2100.32[M+H] +
[0053] Elemental analysis: theoretical value / measured value, C (22.88 / 22.91), H (2.02 / 2.11), I (60.44 / 60.38), N (4.00 / 4.05), O (10.67 / 10.55).
Embodiment 3
[0054] Example 3: Decaiodool-103
[0055]
[0056] Add 10ml of thionyl chloride into the reaction flask, lower the temperature to 0°C, then add 3.3g of 2,3,5,6-tetraiodoterephthalic acid, after the addition, raise the temperature to 50°C and stir for 2 hours. After the reaction, evaporate Thionyl chloride was dissolved in 100ml ethyl acetate for later use.
[0057] 5-amino-N 1 -(2,3-Dihydroxypropyl)-N 3 Dissolve 7.7g of -(2,3-dihydroxypropyl)-2,4,6-triiodo-isophthalamide in 200ml of ethyl acetate, add 2ml of triethylamine, and add the solution obtained in the previous step dropwise under stirring Solution, after the dropwise addition, warm up to reflux and continue to stir for 2h, filter while it is hot, remove the solvent by rotary evaporation of the filtrate, adjust the pH to 2-3 with 4N hydrochloric acid solution, stir for 10min, stand for crystallization for 3h, then recrystallize with ethanol, and filter , and the filter cake was dried under vacuum to obtain 7.6 g o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com