Phenothiazine derivative as well as preparation method and application thereof
A technology of phenothiazine derivatives and derivatives, applied in the field of biomedicine, to achieve the effects of good selectivity, good affinity, and good water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
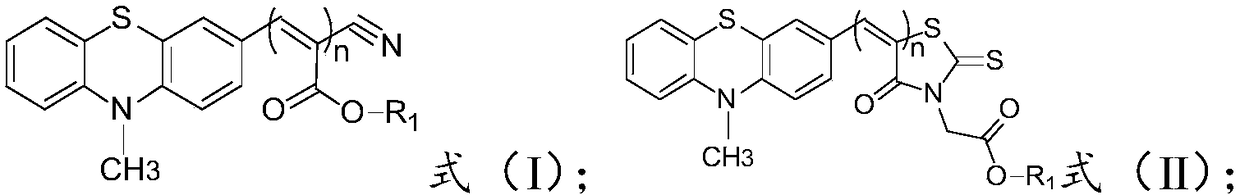
[0042] The invention provides a preparation method of phenothiazine derivatives, comprising:
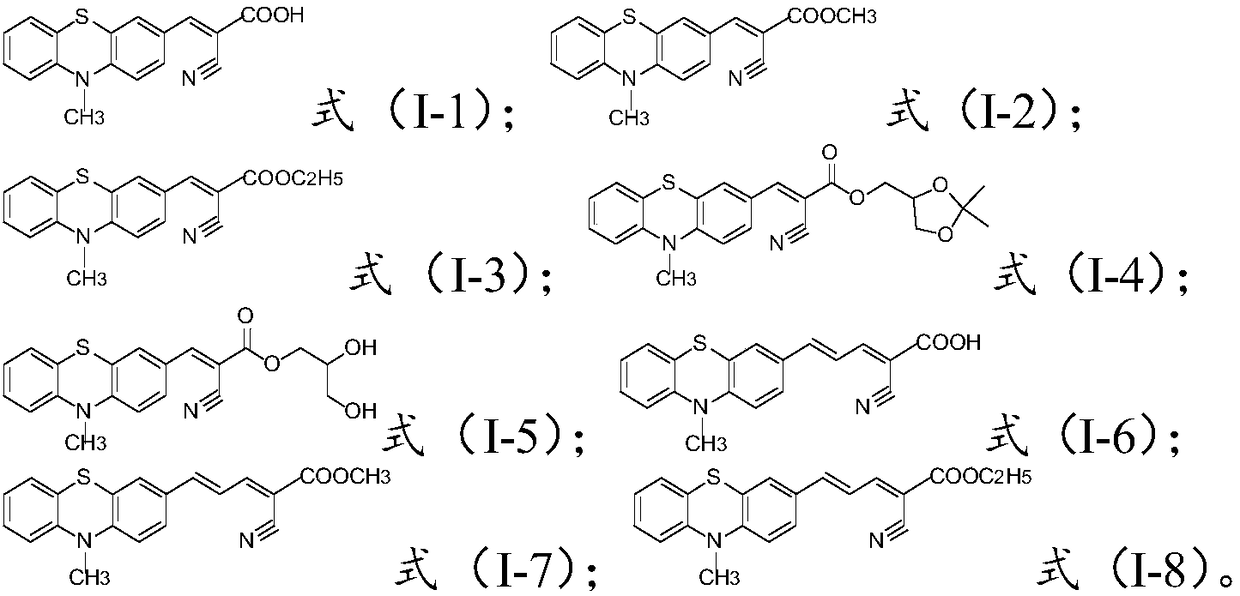
[0043]The thiophenealdehyde compound is condensed with cyanoacetic acid or derivatives thereof to obtain a compound of formula (I); the thiophenealdehyde compound has a structure shown in formula (III) or formula (IV); the cyanoacetic acid Or its derivative is selected from the one in cyanoacetic acid, methyl cyanoacetate, ethyl cyanoacetate and the compound of formula (V) structure;
[0044]
[0045] or
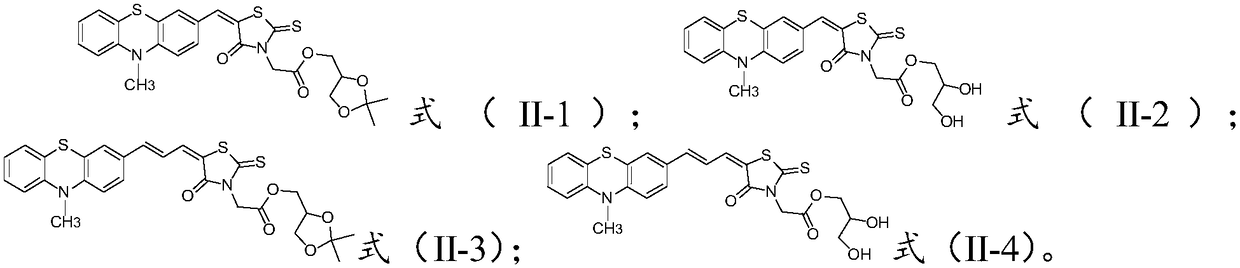
[0046] The thiophene aldehyde compound is subjected to condensation reaction with the compound of the formula (VI) to obtain the compound of the formula (II);
[0047]
[0048] The preparation method of the compound of formula (I) structure provided by the invention is specifically:
[0049] The thiophene aldehyde compound is condensed with cyanoacetic acid or its derivatives to obtain the compound with the structure of formula (I).
Embodiment 1
[0088] Embodiment 1 Synthesis of 10-methylphenothiazine 1
[0089] Weigh NaH (60% purity, 0.5g, 12.55mmol) and slowly add it into a 50mL round bottom bottle with DMF (10mL) in an ice-water bath, then add methyl iodide (0.65g, 5.52mmol) and compound phenothiazine (1g , 5.0mmol), transferred to room temperature and stirred for 2h, TLC monitored the reaction, added water, extracted with DCM (50mL x 3), dried over magnesium sulfate and then concentrated to dryness, separated by silica gel column to obtain product 1 (1.0g), white solid , melting point: 96°C, yield 93.9%.
[0090] 1 H NMR (400MHz, (CD 3 ) 2 CO)δ7.21(td, J=8.0,1.5Hz,2H),7.21(d,J=2.0Hz,1H),7.14(d,J=1.5Hz,1H),6.96-6.93(m,4H) ,3.39(s,3H).
Embodiment 2
[0091] Example 2 Synthesis of 10-methyl-10H-phenothiazine-3-formaldehyde 2
[0092]In an ice-water bath at 0°C, slowly drop phosphorus oxychloride (1.98g, 1.18mL, 12.9mmol) into a 25mL round-bottomed bottle containing dry DMF (856mg, 11.73mmol), stir for 0.5h, then add Compound 1 (500 mg, 2.34 mmol) in 5 mL DCM, the reaction was warmed to reflux for 7 h. After the reaction was monitored by TLC, water was added, extracted with DCM (50mL x 3), dried over magnesium sulfate and then concentrated to dryness. The product 2 (298mg) was obtained by silica gel column separation, a yellow solid, melting point: 106°C, yield 54%.
[0093] 1 H NMR (400MHz, CDCl 3 )δ9.78(s,1H),7.64(dd,J=8.7,2.0Hz,1H),7.59(d,J=2.0Hz,1H),7.17(td,J=7.8,1.6Hz,1H), 7.11(dd, J=7.8,1.6Hz,1H),6.97(t,J=7.5Hz,1H),6.85(d,J=7.5Hz,1H),6.82(d,J=7.5Hz,1H), 3.41(s,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com