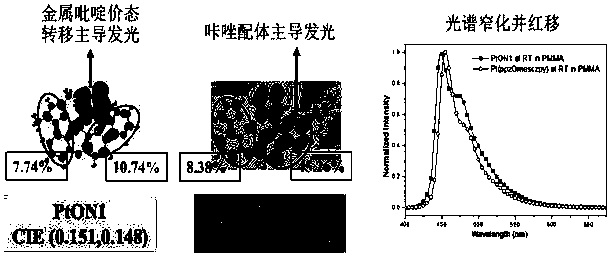
Aryl-substituted tetradentate ligand-coordinated platinum complexes as well as synthesis method and application thereof
A tetradentate ligand, platinum complex technology, applied in the direction of platinum group organic compounds, platinum group organic compounds, compounds containing elements of group 8/9/10/18 of the periodic table, etc. Sexual characteristics and other issues, to achieve the effect of high phosphorescence luminescence intensity, strong rigidity, and energy reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
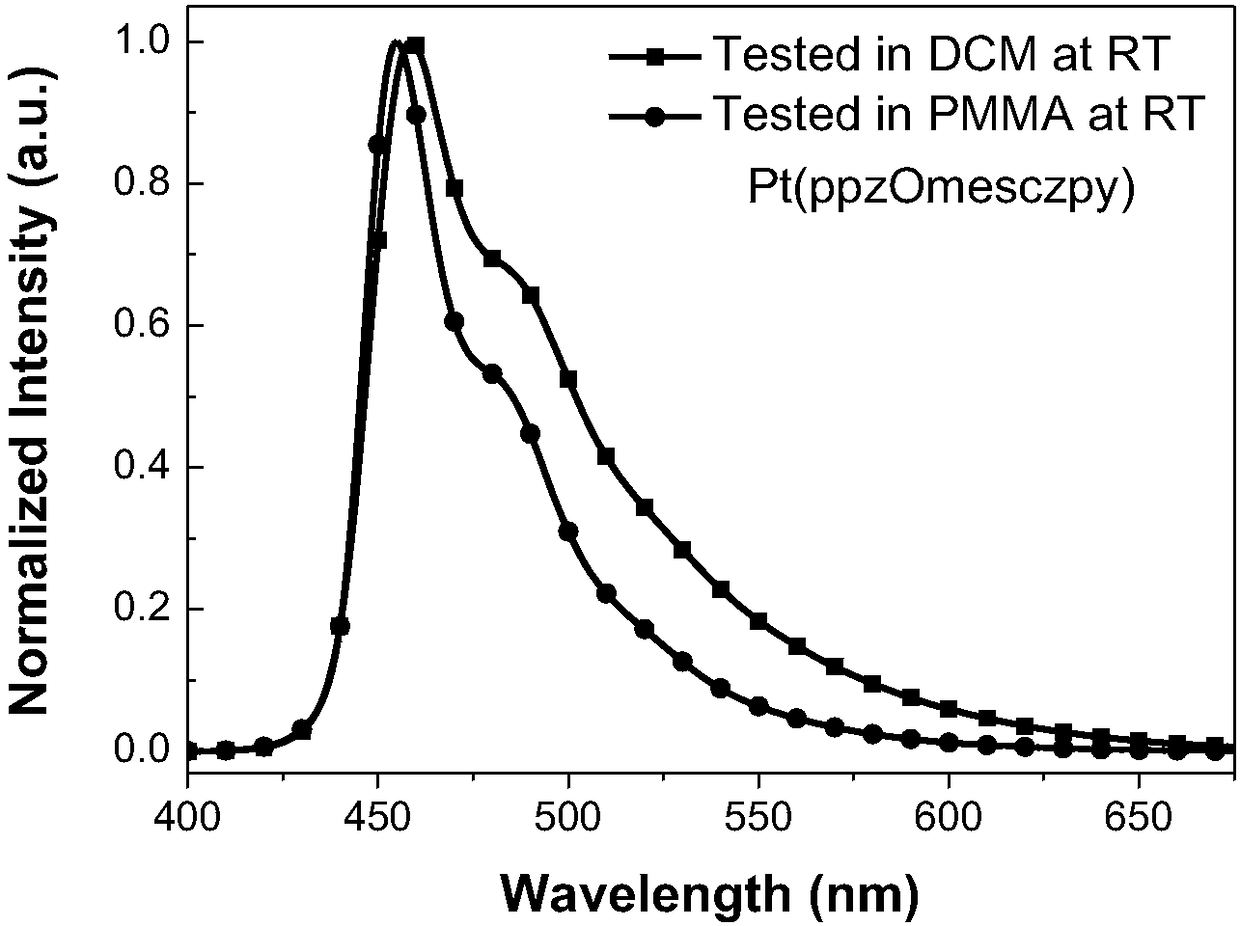
[0046] Embodiment 1. Platinum complex Pt (ppzOmesczpy) preparation method
[0047]
[0048] Synthesis of Pt(ppzOmesczpy) ligand: the chemical equation is shown above, and 2-bromo-7-[3-(1-pyrazolyl)phenoxy]-9-(2-pyridine base) carbazole (193mg, 0.4 mmol), 2,4,6-trimethylphenylboronic acid (131mg, 0.8mmol), S-phos (13mg, 0.03mmol), Pd 2 (dba) 3 (18mg, 0.02mmol), potassium phosphate trihydrate (533mg, 2mmol) and toluene (2mL). The mixture was purged of nitrogen three times and refluxed overnight. Cool to room temperature, add water to quench the reaction, extract with ethyl acetate, combine the organic phases, wash with an appropriate amount of saturated aqueous sodium chloride solution and dry over anhydrous sodium sulfate. The solvent was removed by distillation under pressure, and the obtained crude product was separated and purified by silica gel column chromatography, eluent: petroleum ether / ethyl acetate=10:1, and a white solid was obtained, namely Pt(ppzOmesczpy) lig...
Embodiment 2
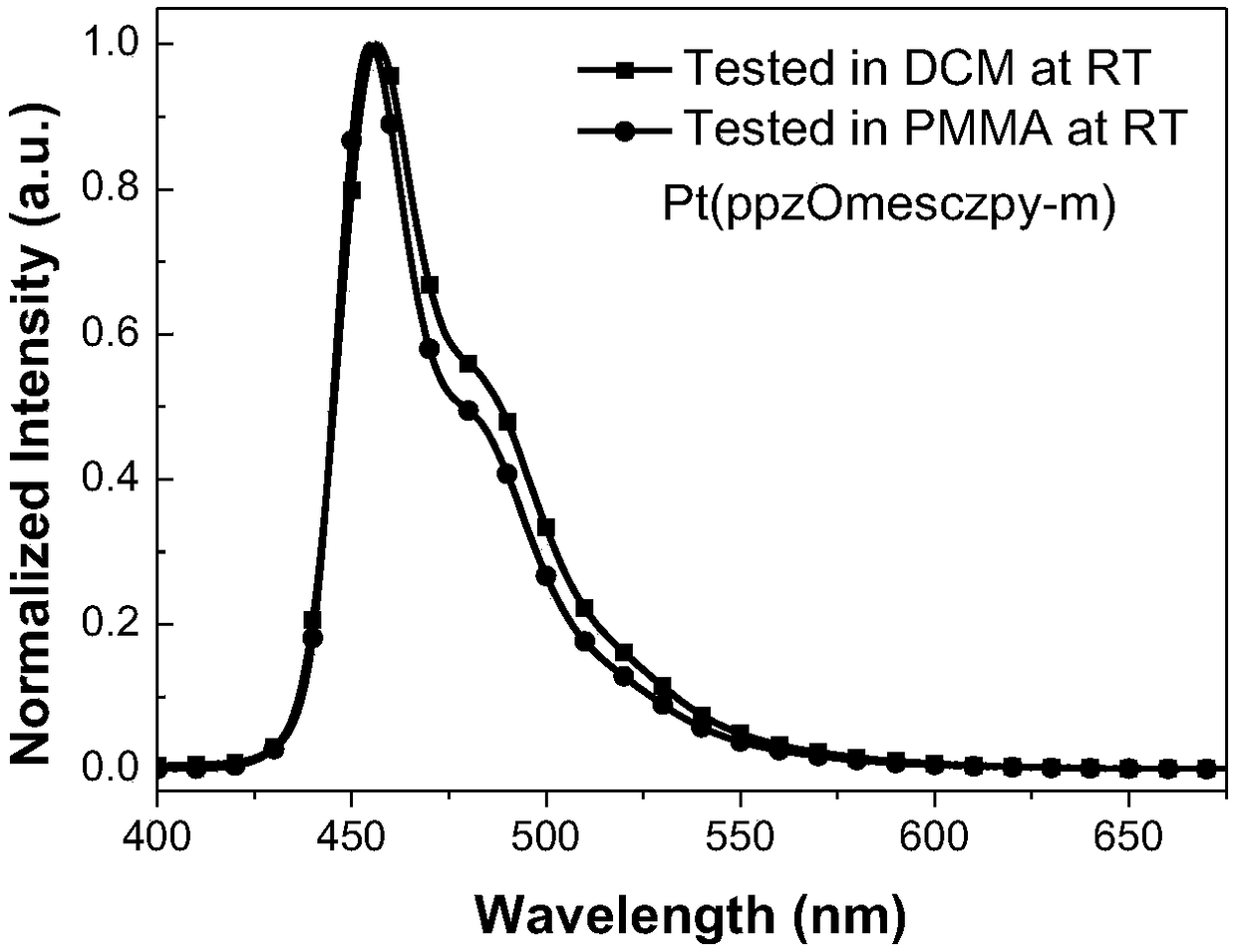
[0051] Embodiment 2. Platinum complex Pt (ppzOmesczpy-m) preparation method
[0052]
[0053] Synthesis of Pt(ppzOmesczpy-m) ligand: the chemical equation is shown above, and 2-bromo-7-[3-(1-pyrazolyl)phenoxy]-9-(4 -Methyl-2-pyridyl)carbazole (71 mg, 0.14mmol), 2,4,6-trimethylphenylboronic acid (46mg, 0.28mmol), S-phos (5mg, 0.01 mmol), Pd 2 (dba) 3 (6mg, 0.0007mmol), potassium phosphate trihydrate (186mg, 0.7mmol) and toluene (1mL). The mixture was purged of nitrogen three times and refluxed overnight. Cool to room temperature, add water to quench the reaction, extract with ethyl acetate, combine the organic phases, wash with an appropriate amount of saturated aqueous sodium chloride solution and dry over anhydrous sodium sulfate. The solvent was removed by distillation under pressure, and the obtained crude product was separated and purified by silica gel column chromatography, eluent: petroleum ether / ethyl acetate=10:1, and 53 mg of Pt(ppzOmesczpy-m) ligand was obtain...
Embodiment 3
[0056] Embodiment 3. Platinum complex Pt (ppzOmesczpy-3m) preparation method
[0057]
[0058] Synthesis of Pt(ppzOmesczpy-3m) ligand: Add 2-bromo-7-[3-(3,5-dimethyl-1-pyrazolyl)phenoxy]-9- (4-Methyl-2-pyridyl)carbazole (262mg, 0.5mmol), 2,4,6-trimethylphenylboronic acid (164mg, 1mmol), S-phos (16mg, 0.04mmol), Pd 2 (dba) 3 (23mg, 0.025mmol), potassium phosphate trihydrate (666mg, 2.5mmol) and toluene (1.5 mL). The mixture was purged of nitrogen three times and refluxed overnight. Cool to room temperature, add water to quench the reaction, extract with ethyl acetate, combine the organic phases, wash with an appropriate amount of saturated aqueous sodium chloride solution and dry over anhydrous sodium sulfate. The solvent was removed by distillation under pressure, and the obtained crude product was separated and purified by silica gel column chromatography, eluent: petroleum ether / ethyl acetate=10:1, and a white solid was obtained, namely, 201 mg of Pt(ppzOmesczpy-3m) li...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com