Method for preparing cyclohexene
A technology of cyclohexene and dicyclohexyl ether, applied in the field of preparation of cyclohexene, can solve the problems of many by-products, complicated separation process, low conversion rate, etc., and achieve the effect of reducing combustion emissions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The preparation method of cyclohexene of one embodiment, comprises the following steps S110~S140:
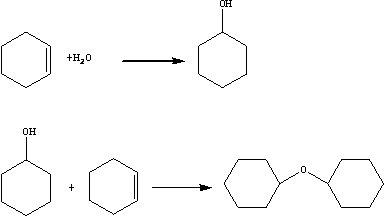
[0030] S110, providing dicyclohexyl ether.
[0031] In this embodiment, dicyclohexyl ether is prepared by the following method:
[0032] (1) Fuel oil is provided, which is produced during the preparation of cyclohexanol by cyclohexene hydration.
[0033] Specifically, the fuel oil contains cyclohexane, methylcyclopentane, cyclohexene, cyclohexanol and dicyclohexyl ether.
[0034] (2) Rectifying and purifying the above-mentioned fuel oil to obtain dicyclohexyl ether.
[0035] Specifically, dicyclohexyl ether can be obtained by sequentially performing normal-pressure rectification and negative-pressure rectification on the above-mentioned fuel oil.
[0036] Further, the conditions of atmospheric distillation are: top temperature 70°C~90°C, reflux ratio 3~10:1.
[0037] The conditions of negative pressure distillation are: pressure 0.1KPa~5KPa, reflux ratio 3~10:1, top t...
Embodiment 1
[0053] The fuel oil containing 1% methylcyclopentane, 15% cyclohexane, 5% cyclohexene, 35% cyclohexanol and 44% dicyclohexyl ether is sequentially subjected to atmospheric distillation and negative pressure Rectification, normal pressure rectification to obtain the light component fraction section, negative pressure rectification to obtain the cyclohexanol fraction section and the dicyclohexyl ether fraction section respectively. Among them, the content of cyclohexanol in the cyclohexanol fraction section is >99.5%, and the content of dicyclohexyl ether in the dicyclohexyl ether fraction section is 87%.
[0054] React the dicyclohexyl ether fraction above in a fixed-bed reactor equipped with a potassium carbonate catalyst at a reaction temperature of 280°C and a space velocity of 0.2h -1 , the reaction pressure is 0MPa, and the cleavage product is obtained.
[0055] Cool the cracked product above to below 60°C, separate the oil from water, take the oil phase for rectification...
Embodiment 2
[0067] The fuel oil containing 1% methylcyclopentane, 15% cyclohexane, 5% cyclohexene, 35% cyclohexanol and 44% dicyclohexyl ether is sequentially subjected to atmospheric distillation and negative pressure Rectification, normal pressure rectification to obtain the light component fraction section, negative pressure rectification to obtain the cyclohexanol fraction section and the dicyclohexyl ether fraction section respectively. Among them, the content of cyclohexanol in the cyclohexanol fraction section is >99.5%, and the content of dicyclohexyl ether in the dicyclohexyl ether fraction section is 87%.
[0068] React the dicyclohexyl ether fraction above in a fixed-bed reactor equipped with a 4A molecular sieve catalyst at a reaction temperature of 330°C and a space velocity of 0.3h -1 , the reaction pressure is 0.2MPa, and the cracked product is obtained.
[0069] Cool the cracked product above to below 60°C, separate the oil from water, take the oil phase for rectification...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com