Full continuous flow synthesis process for 2-methyl-1,4-naphthoquinone
A fully continuous, flow synthesis technology, applied in the field of chemistry, can solve the problems of unstable product quality, large consumption of oxidant, low production efficiency, etc., and achieve the effect of shortening the total reaction time, reducing excessive oxidation and improving production efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0096] Embodiment 1#~12#
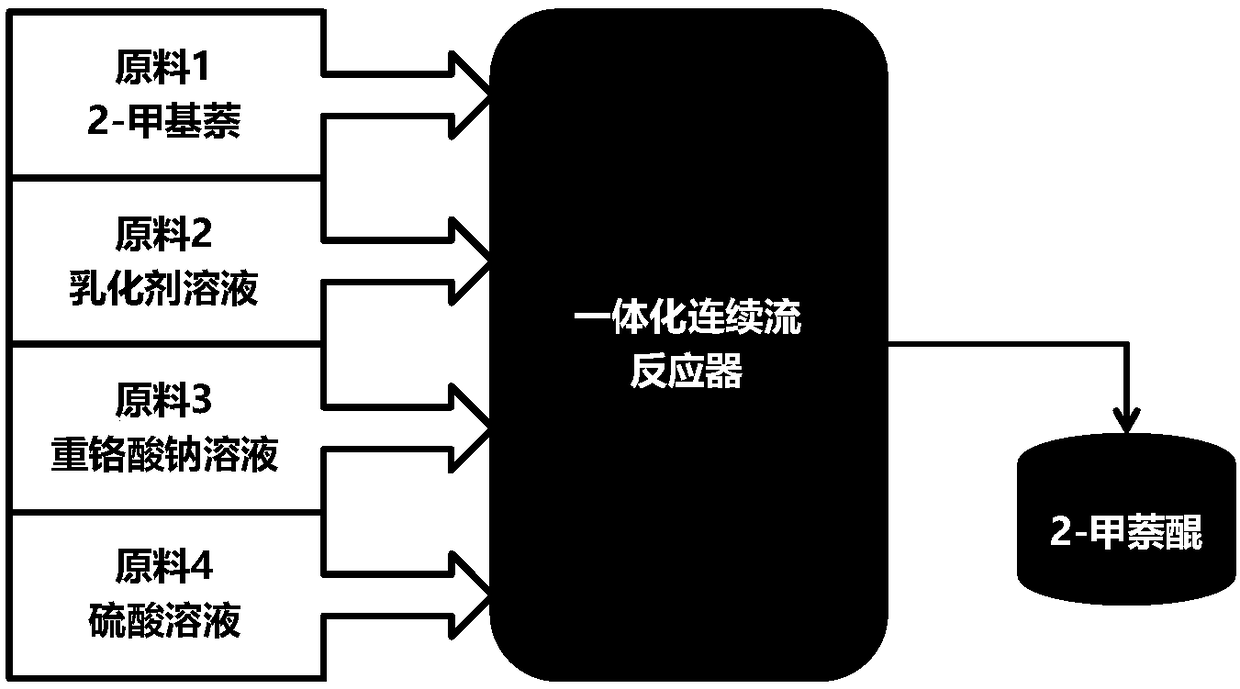
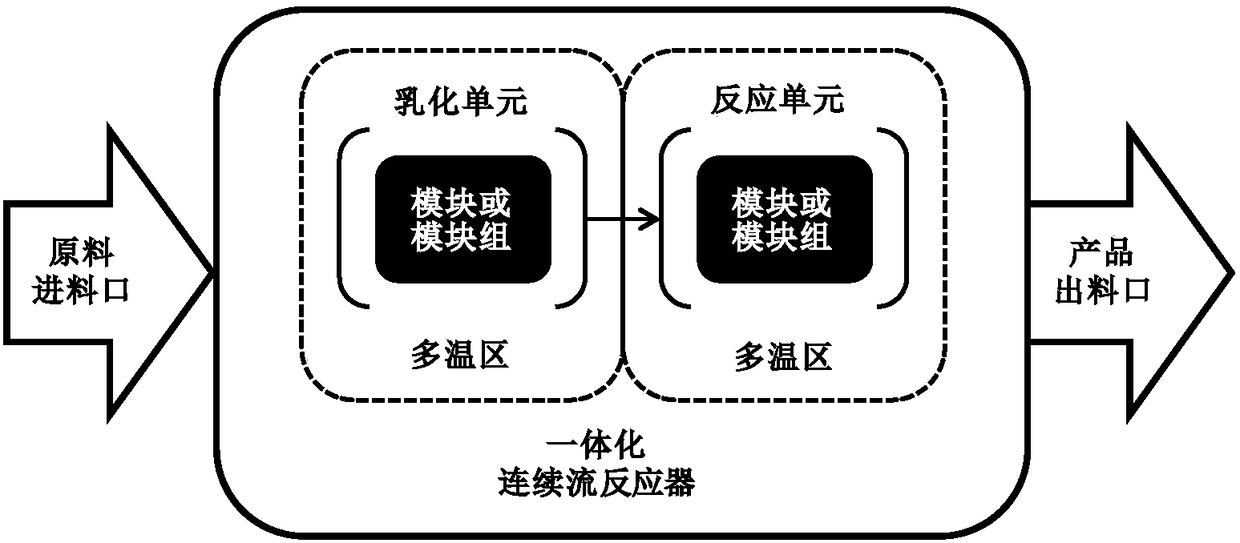
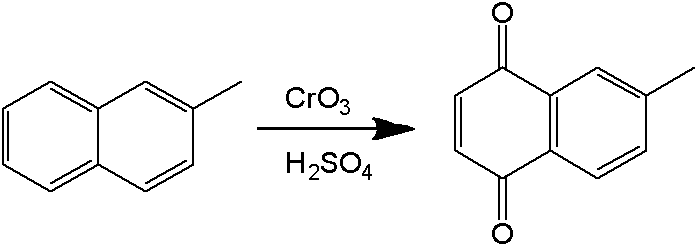
[0097] The molten 2-methylnaphthalene and emulsifier solution are emulsified by a constant flow feed pump. The "dynamic" milk flow after emulsification enters the continuous flow reactor directly without stopping. Sodium dichromate solution and a stream of sulfuric acid solution are also fed into the reactor 2-methylnaphthalene through a constant flow feed pump to react. Emulsification time is RT e , the reaction temperature is T, and the oxidation reaction time is RT r . The formula for calculating the total reaction time is: RT=RT e +RT r .
[0098] where emulsification time RT e The calculation formula is
[0099]
[0100] Oxidation reaction time RT r The calculation formula is similar to the emulsification time calculation.
[0101] Concrete embodiment condition and result are shown in the following table.
[0102]
[0103]
Embodiment 13
[0104] Embodiment 13#~18#
[0105] The molten 2-methylnaphthalene and emulsifier solution are emulsified by a constant flow feed pump. The difference from Examples 1# to 12# is that the sulfuric acid solution is divided into three streams with different concentrations and different flow rates, and the sulfuric acid solution is pumped into different positions of the reactor. The temperature from the first addition of sulfuric acid solution to the second addition of sulfuric acid solution is set as T 1 , the reaction time is RT r1 , the temperature between the addition of sulfuric acid solution for the second time and the addition of sulfuric acid solution for the third time is set to T 2 , the reaction time is Rt r2 , the temperature between the third time sulfuric acid solution is added to the outlet is set to T 3 , the reaction time is RT r3 , the total reaction time is RT, and the calculation formula is RT=RT e +RT r1 +RT r2 +RT r3 . The calculation principle of ea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com