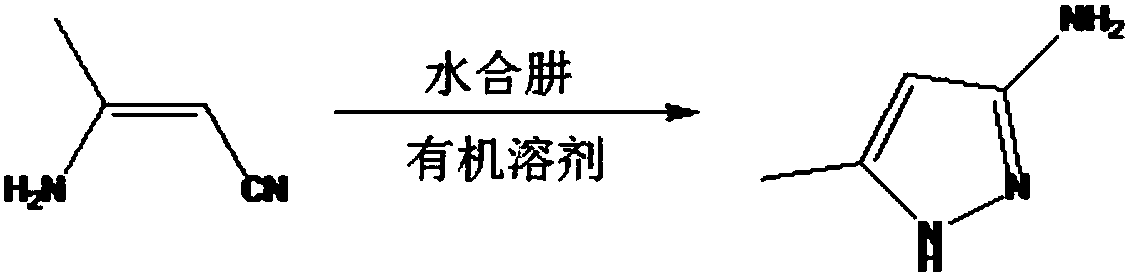
Process for synthesizing 3-amino-5-methylpyrazole
A methylpyrazole and synthetic process technology, which is applied in the field of 3-amino-5-methylpyrazole synthetic process, can solve problems such as easy danger and difficult post-processing, and achieve high purity, mild reaction conditions, and easy synthesis The effect of simple process route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Add 2000g of 3-aminobutene nitrile and 5L of ethanol into a 10L reaction flask, start stirring, stir until it is clear, add 2500g of 80% hydrazine hydrate, stir at room temperature for 10-20min., slowly heat to reflux, reaction temperature 85-95℃ ; Pay attention to a large amount of gas generation; heat preservation reaction for 1.5-2.5 hours, TLC monitoring, the reaction of the raw materials is complete; the reaction liquid is concentrated under reduced pressure at 50-60°C to 20%-30% of the original reaction liquid volume; After cooling, it becomes a pale yellow solid), 2230 g, yield 94.2%, detected by GC and HPLC, the purity of 3-amino-5-methylpyrazole is above 98%, and the single impurities are less than 0.5%.
Embodiment 2
[0021] As in Example 1, only the organic solvent ethanol was changed to the following substances to prepare 3-amino-5-methylpyrazole, and the yield was calculated;
[0022] Group
Embodiment 3
[0024] Same as Example 1, only changing the mass ratio of 3-aminobutenenitrile, organic solvent, and hydrazine hydrate to prepare 3-amino-5-methylpyrazole, and calculate its yield;
[0025] Group
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com