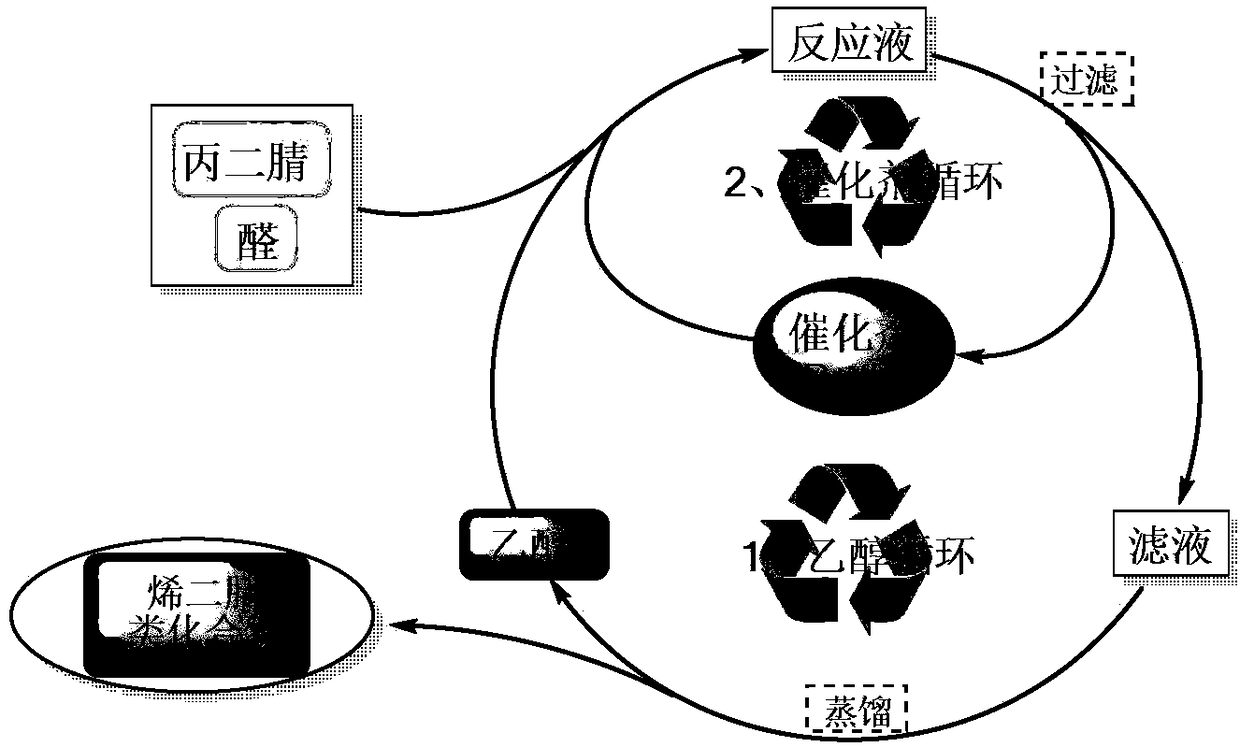
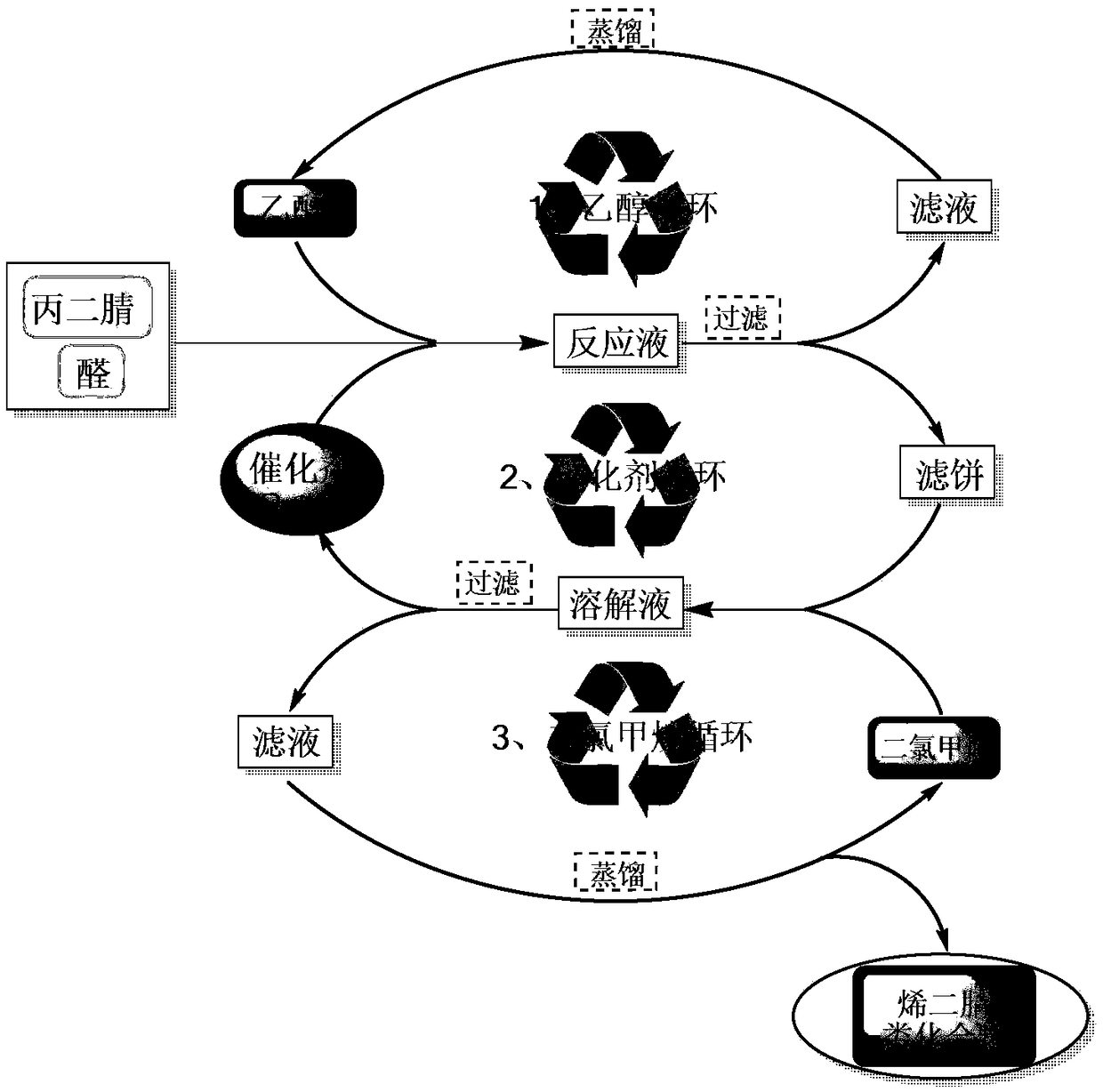
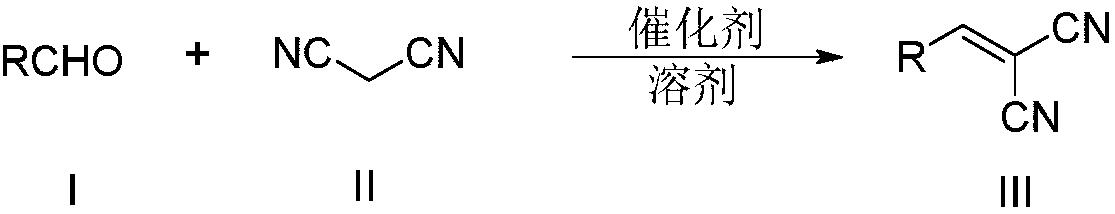
Synthesis method of alkenyl dinitrile compounds
A synthesis method and compound technology are applied in the field of synthesis of enedionitrile compounds, which can solve problems such as a large amount of alkaline waste water, polluted environment and the like, and achieve the effects of high yield, reduced pollution and good purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Preparation of enedionitrile compound (III-1)
[0032] The reaction formula is as follows:
[0033]
[0034] Add 5.306g (50mmol) of benzaldehyde (I-1), 0.5g (Ru molar amount is 5‰ of (I-1)) Ru / C catalyst, 3.303g (50mmol) of malononitrile ( II) With 100 mL of ethanol, the reaction was stirred at room temperature for 12 hours. GC-MS detected that the raw material had reacted completely, and the reaction was stopped. The reaction solution was filtered once to obtain the first filtrate and the first filter cake. The first filtrate was used to recover ethanol by the rotary evaporator; the first filter cake was dissolved with 50 mL of dichloromethane, and the second filter was filtered to obtain the second filtrate and the second filter cake, using 5 mL The second filter cake was washed with dichloromethane and filtered three times to obtain the third filtrate and the third filter cake. The third filter cake is the Ru / C catalyst and can be used in the next batch of rea...
Embodiment 2
[0036] Example 2: Preparation of enedionitrile compound (III-2)
[0037] The reaction formula is as follows:
[0038]
[0039] Add 6.008g (50mmol) o-methylbenzaldehyde (I-2), 0.5g (Ru molar amount is 5‰ of (I-2)) Ru / C catalyst, 3.303g (50mmol) propylene The dinitrile (II) and 100 mL of ethanol were stirred at room temperature for 14 hours, and the following operations were the same as in Example 1. Finally, 8.242 g of solid was obtained, the yield was 98.0%, and the GC-MS purity was 99.0%. The structure of compound formula (III-2) is characterized as follows:
[0040] 1 H-NMR(CDCl 3 ,500MHz):δ8.12(s,1H),8.11-8.09(m,1H),7.38-7.33(m,2H),2.46(s,3H); 13 C-NMR(CDCl 3 ,125MHz):δ158.2,139.8,134.3,131.5,130.0,128.4,127.1,113.9,112.5,84.1,19.9; GC-MS(EI): m / z 168[M + ].
Embodiment 3
[0041] Example 3: Preparation of enedionitrile compound (III-3)
[0042] The reaction formula is as follows:
[0043]
[0044] Add 6.008g (50mmol) of p-methylbenzaldehyde (I-3), 0.5g (Ru molar amount is 5‰ of (I-3)) Ru / C catalyst, 3.303g (50mmol) of propylene into the reaction flask Dinitrile II and 80 mL of ethanol were stirred at room temperature for 10 hours, and the following operations were the same as in Example 1. Finally, 8.326 g of solid was obtained, the yield was 99.0%, and the GC-MS purity was 99.0%. The structure of compound formula (III-3) is characterized as follows:
[0045] 1 H-NMR(CDCl 3 ,500MHz): δ8.20(d,J=8.1Hz,2H), 7.73(s,1H), 7.35(d,J=8.1Hz,2H), 2.47(s,3H); 13 C-NMR(CDCl 3 ,125MHz):δ159.8,146.4,130.9,130.4,128.4,114.0,112.9,81.1,22.0; GC-MS(EI): m / z 168[M + ].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com