Anti-torch-IgM antibody spectrum chip, preparation method of chip and TORCH detection kit
A technology of antibody spectrum and kit, applied in the field of anti-torch-IgM antibody spectrum chip and its preparation, TORCH detection kit, which can solve the problems of high cost, high price, and inability to quantify, and achieve high accuracy and stability Good performance, the effect of reducing the amount of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1, the preparation of anti-torch-IgM type antibody spectrum chip kit
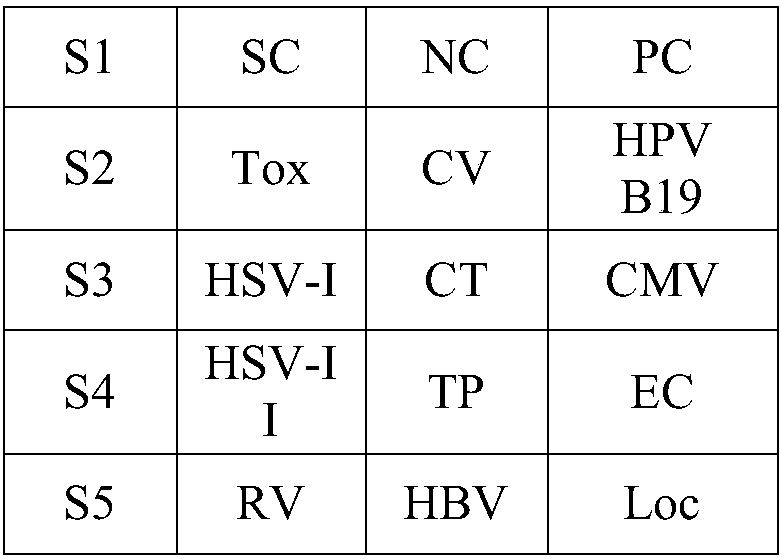
[0049] 1) The array design of the chip and the specific distribution of antigens and reference points are as follows (not limited to the following arrangement design):
[0050]
[0051] 2), specific coating process:
[0052] Antigen and relevant reference point proteins are first diluted as follows:
[0053] PC, NC, S1, S2, S3, S4, S5, and EC points in the array are respectively coated with 2μg / ml, 0.01μg / ml, 0.5μg / ml, 1μg / ml, 2.5μg / ml, 5μg / ml , 10 μg / ml, 2.5 μg / ml human IgM, the dilution buffer is the CB buffer solution of pH9.6 (which contains 0.5% PEG4000, 5% trehalose, 0.02% Captisol, 0.05% Proclin300, and 15 % of glycerol).
[0054] SC dots were coated with 2 μg / ml goat anti-IgM antibody, and the dilution buffer was CB buffer at pH 9.6.
[0055] Loc spots were coated with 2 μg / ml human IgM, and the dilution buffer was CB buffer at pH 9.6.
[0056]...
Embodiment 2
[0070] Example 2, Evaluation of the accuracy of the anti-torch-IgM antibody profile chip kit
[0071] 1. 10 cases of anti-toxoplasma IgM positive serum and 10 cases of anti-toxoplasma IgM negative serum determined by Diasorin company ELISA kit screening were tested with the anti-torch-IgM antibody spectrum chip prepared in Example 1, and the results were shown in Table 1 (+ means positive, - means negative).
[0072] Anti-torch-IgM antibody spectrum chip test anti-toxoplasma gondii IgM serum result prepared by table 1 embodiment 1
[0073]
[0074] 2. Determine anti-rubella virus IgM positive sera and 10 examples of anti-rubella virus IgM negative sera with Diasorin company's ELISA kit screening, and use the anti-torch-IgM antibody spectrum chip test prepared in Example 1, the results are as follows in table 2 ( + means masculine, - means negative).
[0075] The anti-torch-IgM type antibody spectrum chip test anti-rubella virus IgM serum result that table 2 embodiment 1 p...
Embodiment 3
[0100] Embodiment 3, anti-torch-IgM type antibody profile chip kit specificity evaluation
[0101] Compare the specificity of the anti-torch-IgM antibody spectrum chip kit of the present invention with the traditional ELISA kit. Taking the Toxoplasma project as an example, 20 cases of anti-Toxoplasma IgM antibody clinical negative serum were selected, and the anti-torch-IgM antibody spectrum chip prepared in Example 1 was tested simultaneously with the Diasorin ELISA kit. The test data are as follows in Table 11 (“ +" means positive, "±" means weak positive, "-" means negative).
[0102] The anti-torch-IgM type antibody spectrum chip specificity comparison result prepared in Table 11 Example 1
[0103]
[0104] The results showed that the anti-torch-IgM antibody spectrum chip prepared in Example 1 had no false positives in the 20 negative sera tested, while 2 false positives occurred in the 20 negative sera tested by the Diasorin ELISA kit on the market. It shows that the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com