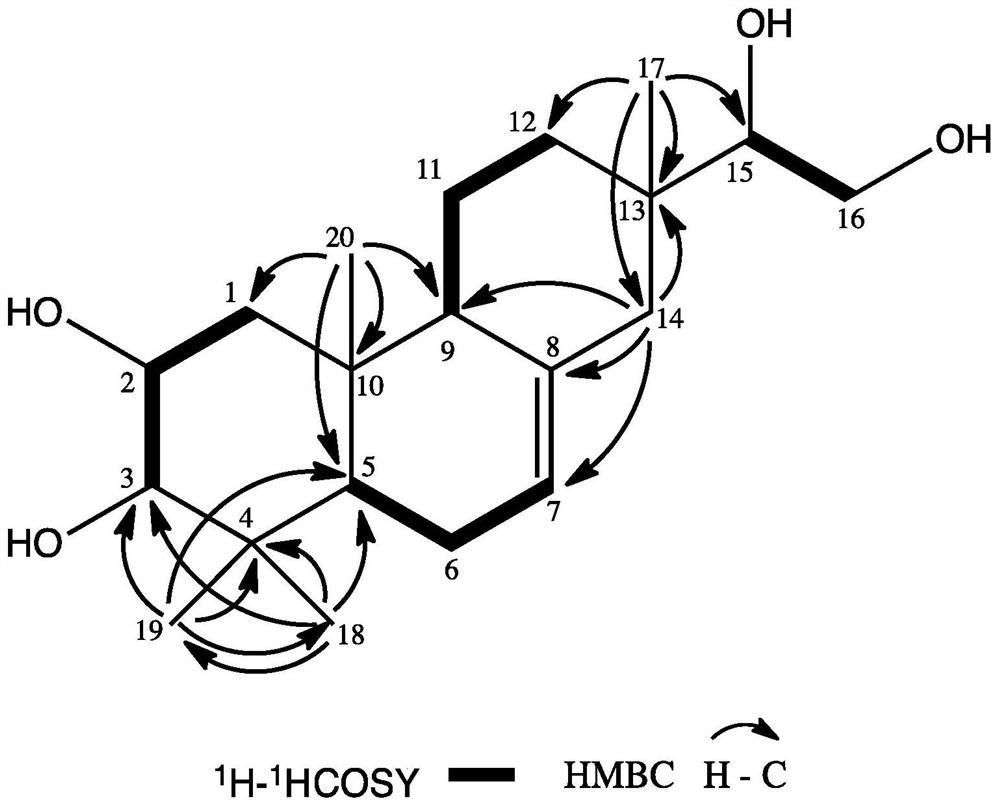
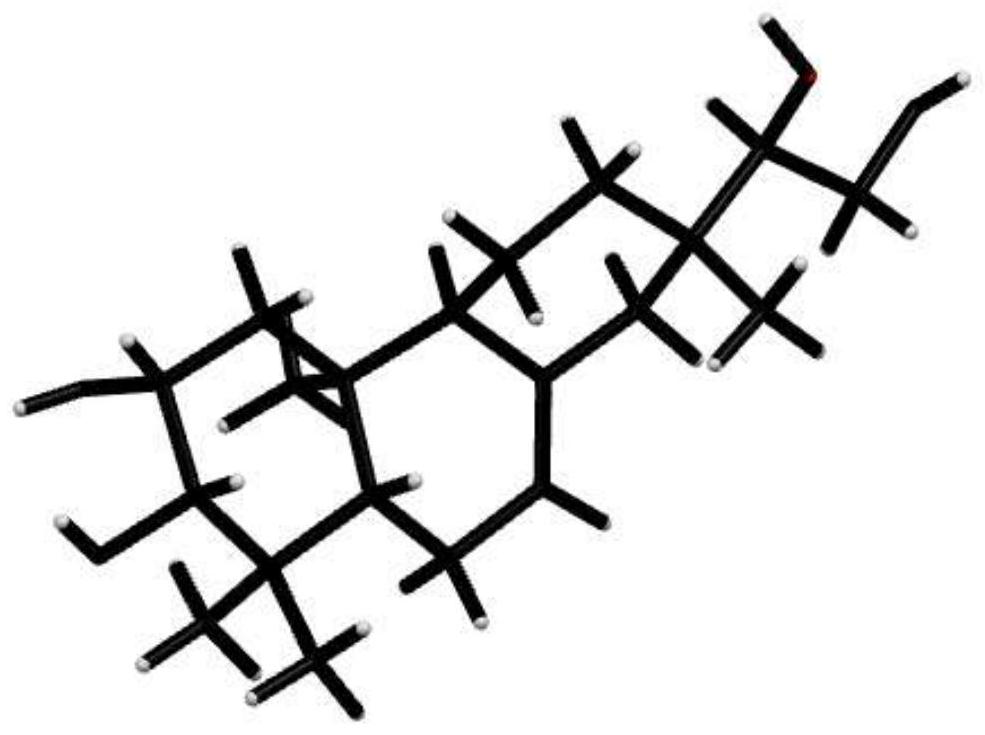
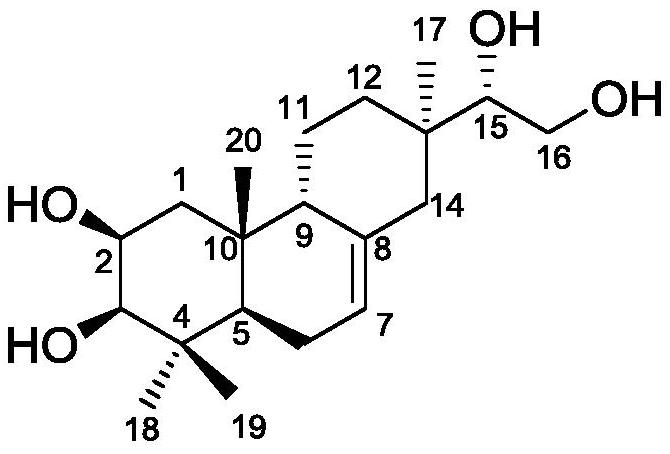
A kind of honeysuckle diterpenoid compound and its preparation method and anti-agricultural fungus use
A technology of compounds and diterpenoids, applied in the field of natural products, can solve the problems of endangering human and animal health, poor selectivity, long residual time, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Example 1: Cut 5 kg of dried Lonicera pilosulae into 0.2-0.3 cm sections, add 95% ethanol with 6 times the volume of the crude drug, reflux extraction twice, each time for 2 hours, combine the crude extracts, and decompose at 70 ° C. Concentrate under reduced pressure to 0.5 times the volume of the medicinal material, then add petroleum ether and ethyl acetate in sequence according to the volume of 1:2 to extract 3 times respectively, combine the petroleum ether and ethyl acetate extracts, concentrate under reduced pressure, and obtain 14 g of petroleum ether extract and acetic acid Ethyl ester extract 200g. Get the ethyl acetate extract, use silica gel column chromatography to segment, and use petroleum ether-ethyl acetate (20:1), petroleum ether-ethyl acetate (10:1), petroleum ether-ethyl acetate (4:1 ), petroleum ether-ethyl acetate (1:1) solution, combined eluents with similar components, and concentrated to obtain 4 fractions of F2-1 to F2-4. Then, by comprehensiv...
Embodiment 2
[0022] Example 2: Cut 5 kg of dried Lonicera chinensis roots into 0.2-0.3 cm sections, add 5 times the volume of crude drug methanol, reflux extraction for 3 times, each time for 1.5 h, combine the crude extracts, and depressurize at 50 ° C Concentrate to 0.5 times the volume of the medicinal material, then add petroleum ether and ethyl acetate in sequence according to the volume of 1:1 to extract 3 times respectively, combine the petroleum ether and ethyl acetate extracts, concentrate under reduced pressure, and obtain 16 g of petroleum ether extract and ethyl acetate Ester extract 220g. Using methods such as silica gel column chromatography, gel LH-20 column chromatography, and recrystallization, the compound Lonicera cinereus (160 mg) was isolated from the ethyl acetate extract, with a yield of 0.0032%, UPLC- The purity of the product detected by HRESI-MS was 98.8%.
Embodiment 3
[0023] Example 3: Cut 10 kg of dried Lonicera pilosula root into 0.2-0.3 cm sections, add 80% methanol / water 6 times the volume of the crude drug, reflux extraction twice, each time for 2 hours, combine the crude extracts, 50 Concentrate under reduced pressure at ℃ to 1.5 times the volume of the medicinal material, then add petroleum ether and ethyl acetate to extract 5 times in sequence at a volume of 1:2, combine petroleum ether and ethyl acetate extracts, concentrate under reduced pressure, and obtain petroleum ether extract and Ethyl acetate extract. Using methods such as silica gel column chromatography, gel LH-20 column chromatography, and recrystallization, the compound Lonicera cinereus (315 mg) was isolated from the ethyl acetate extract, with a yield of 0.00315%, UPLC- The purity of the product detected by HRESI-MS was 98.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com