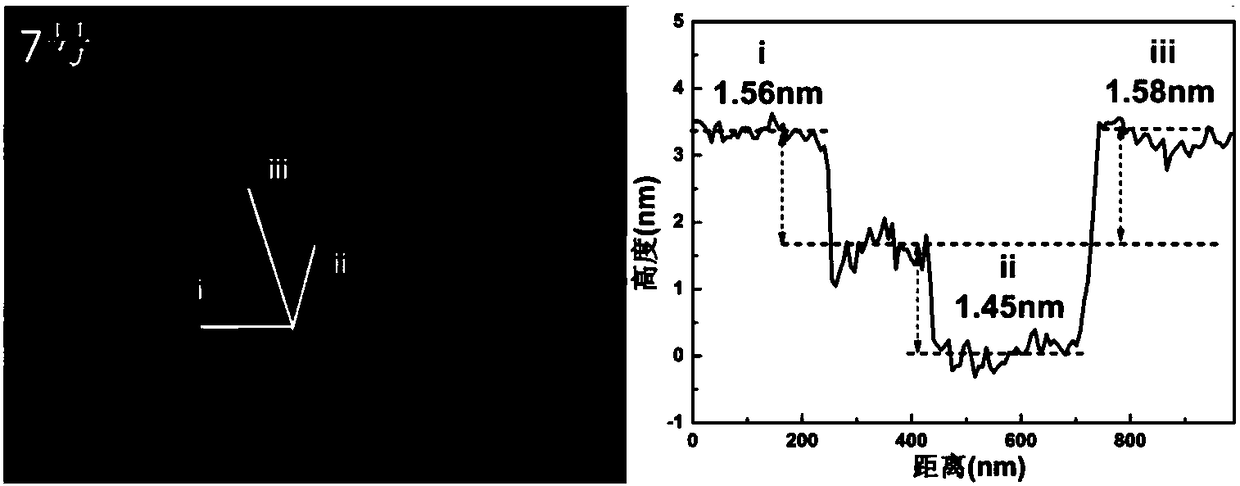
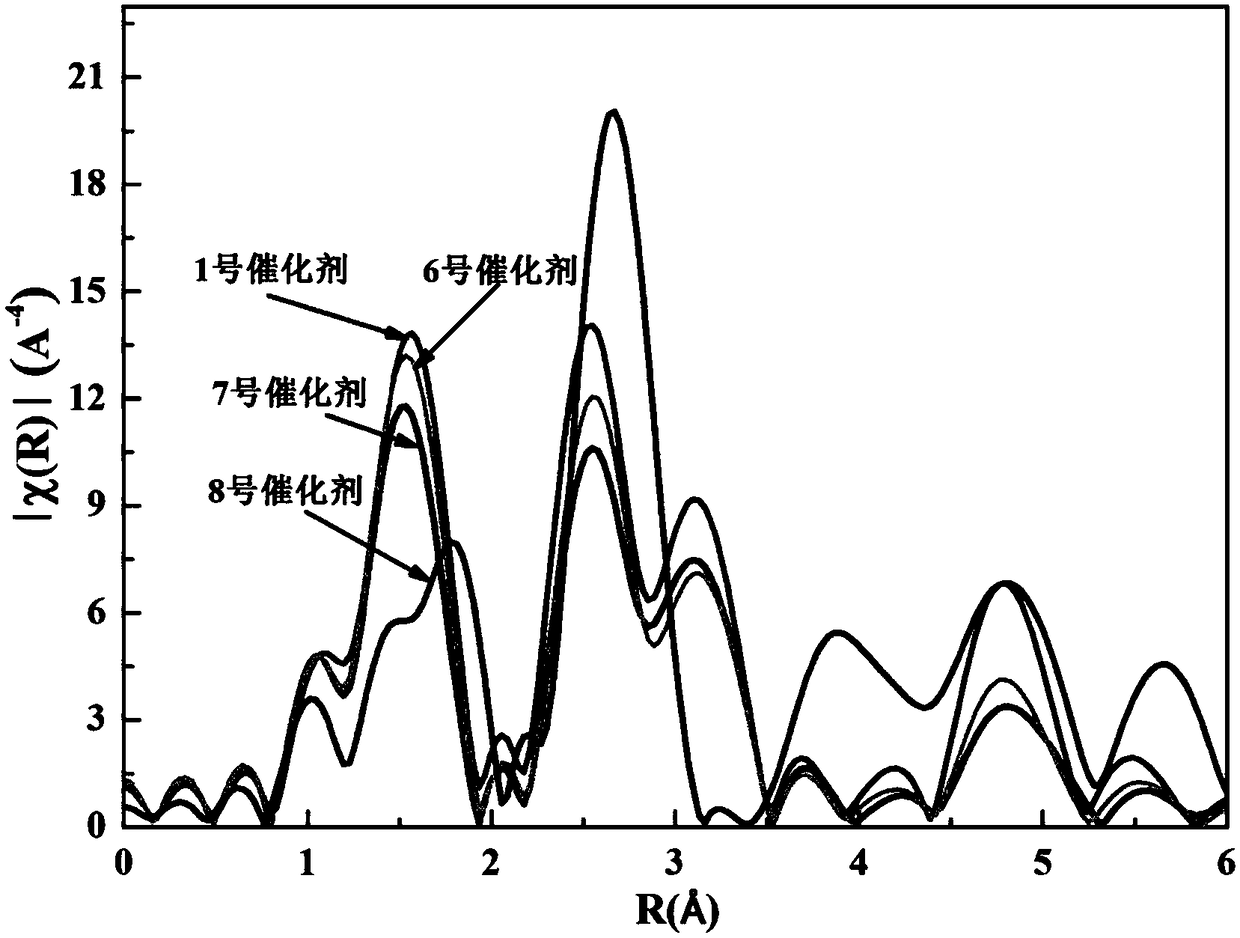
Oxygen defect-rich ultrathin nickel cobalt oxide nanosheet electrode array and preparation method thereof
A nickel-cobalt oxide and nanosheet array technology, which is applied in nanotechnology, electrodes, and nanotechnology for materials and surface science, can solve problems such as high overpotentials, increase the number, and improve oxygen evolution and oxygen reduction reactions Catalytic activity, the effect of increasing the electrochemical specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 11
[0028] The preparation of embodiment 1 No. 1 catalyst
[0029] 1) Dissolve 1mmol of cobalt nitrate, 5mmol of ammonium fluoride and 15mmol of urea in 80mL of ultrapure water, and dissolve with electromagnetic stirring to obtain a clear solution.
[0030] 2) The solution obtained in the above step 1) was transferred to a hydrothermal kettle, reacted in a constant temperature oven at 150°C for 5 hours, cooled naturally to room temperature, and the product was washed and dried to obtain the No. 1 catalyst prepolymer.
[0031] 3) Put the No. 1 catalyst prepolymer into a muffle furnace, heat-treat at 300° C. for 1-4 hours, and obtain No. 1 catalyst.
Embodiment 2
[0032] Preparation of No. 2 catalyst of embodiment 2
[0033] 1) Immerse the nickel foam in absolute ethanol, ultrasonically clean it for 0.5-1 hour, and let it dry naturally.
[0034] 2) Dissolve 1mmol of cobalt nitrate, 5mmol of ammonium fluoride and 15mmol of urea in 80mL of ultrapure water, and dissolve with electromagnetic stirring to obtain a clear solution.
[0035] 3) Transfer the solution obtained in the above step 2) to a hydrothermal kettle, immerse the nickel foam cleaned in step 1) in the solution, react in a constant temperature oven at 150°C for 5 hours, cool naturally to room temperature, and take out the nickel foam, After washing and drying, No. 2 catalyst prepolymer was obtained.
[0036] 4) Put the No. 2 catalyst prepolymer into a muffle furnace, heat-treat at 300° C. for 1-4 hours, and obtain No. 2 catalyst.
Embodiment 3
[0037] Preparation of No. 3 catalyst of embodiment 3
[0038] 1) Immerse the nickel foam in absolute ethanol, ultrasonically clean it for 0.5-1 hour, and let it dry naturally.
[0039] 2) Dissolve 1 mmol of cobalt nitrate, 5 mmol of ammonium fluoride, 15 mmol of urea and 0.5 mmol of silver nitrate in 80 mL of ultrapure water, and dissolve with electromagnetic stirring to obtain a clear solution.
[0040] 3) Transfer the solution obtained in the above step 2) to a hydrothermal kettle, immerse the nickel foam cleaned in step 1) in the solution, react in a constant temperature oven at 150°C for 5 hours, cool naturally to room temperature, and take out the nickel foam, After washing and drying, catalyst prepolymer No. 3 was obtained.
[0041] 4) Put the No. 3 catalyst prepolymer into a muffle furnace, heat-treat at 300° C. for 1-4 hours, and obtain No. 3 catalyst.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com