Method for extracting copper ions in wastewater through ionic liquid
An ionic liquid and copper ion technology, applied in the direction of improving process efficiency, can solve the problems of low extraction efficiency, secondary pollution, unstable ionic liquid and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
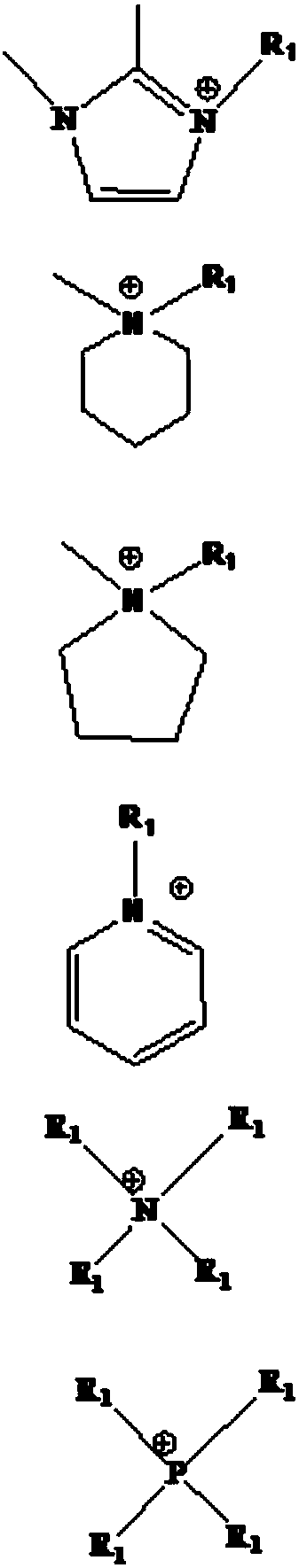
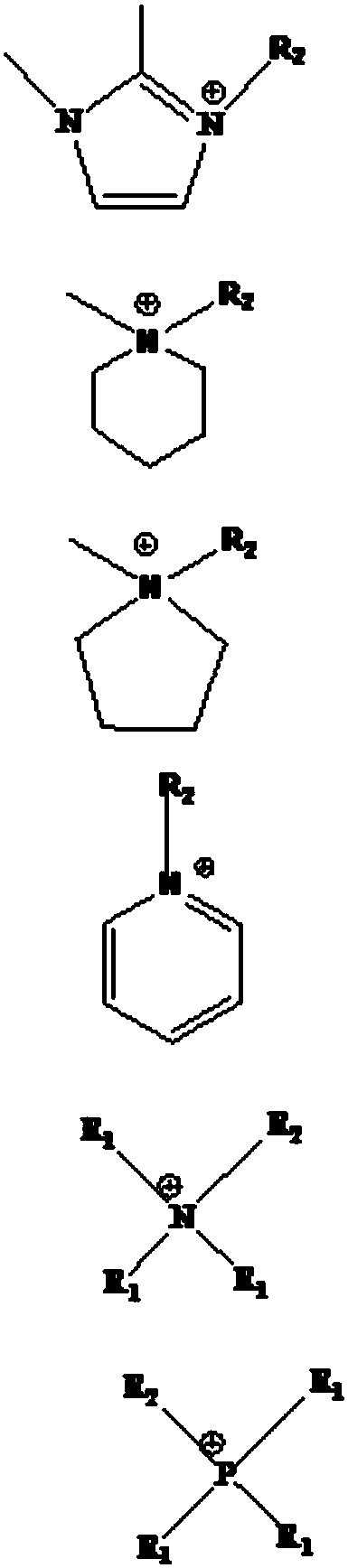
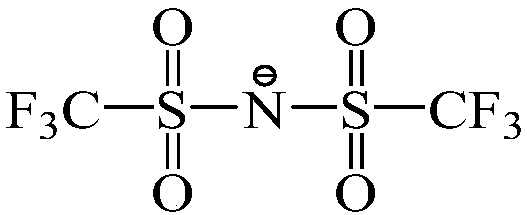
Image
Examples
Embodiment 1
[0035] The ionic liquid used in this embodiment is 1-butyl-3-methylimidazole bis(trifluoromethylsulfonyl)imide [BMIM][NTf 2 ] extract the method for the copper ion in waste water, carry out according to the following steps:
[0036] Step 1, take 6mL of waste water containing divalent copper ions as a raw material solution and place it in a centrifuge tube, the concentration of divalent copper ions in the raw material solution is 3ug / mL, the pH of the raw material solution=8, and then add 1300uL of 0.2g / L 2 -Pyridine formaldehyde oxime (2-PA) solution, 225uL ionic liquid [BMIM][NTf 2 ] in a centrifuge tube, shake at room temperature 25°C and extract for 6 minutes;
[0037] Step 2, the solution after the extraction in step 1 is carried out 1000r centrifugation, the time of centrifugation is 4min, after separating, get the supernatant liquid, measure the divalent copper ion content in the aqueous phase before and after its extraction with flame atomic absorption spectrophotomete...
Embodiment 2
[0049] The ionic liquid used in this embodiment is 1-butyronitrile-3-methylimidazole bistrifluoromethanesulfonic acid imide [PCNMMIM][NTf 2 ] extract the method for the copper ion in waste water, carry out according to the following steps:
[0050] Step 1, take 6mL of waste water containing divalent copper ions as the raw material solution and place it in a centrifuge tube. The concentration of divalent copper ions in the raw material solution is 3ug / mL, the pH of the raw material solution is 7, and then add 1500uL 0.5g to the centrifuge tube / L of sodium diethyldithiocarbamate (DDTC) solution, 250uL selected ionic liquid [PCNMMIM][NTf 2 ], and extracted by shaking at room temperature 28°C for 6 min.
[0051] Chelating agent solution Preparation of sodium diethyldithiocarbamate (DDTC) solution: Weigh 0.5g of DDTC, add water to dissolve it, and set the volume in a 100mL volumetric flask to make a 5g / L DDTC solution. The standard working solution is used step by step dilution....
Embodiment 3
[0055] The present embodiment adopts ionic liquid 1-butyronitrile-3-methylimidazole bistrifluoromethanesulfonate imide [PCNMMIM][NTf 2 ] extract the method for the copper ion in waste water, carry out according to the following steps:
[0056] Step 1, take 6mL of waste water containing divalent copper ions as the raw material solution and place it in a centrifuge tube. The concentration of divalent copper ions in the raw material solution is 5ug / mL, the pH of the raw material solution is 4, and then add 1300uL 0.1g to the centrifuge tube / L of 1-(2-pyridylazo)-2-naphthol solution, 225uL selected ionic liquid [PCNMMIM][NTf 2 ], shake and extract at room temperature 30°C for 10 min.
[0057] Step 2, the solution after the extraction of step 1 is subjected to 3000r centrifugation, and the centrifugation time is 4min. After the separation, the supernatant is taken, and the content of divalent copper ions in the aqueous phase before and after the extraction is measured with a flam...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com