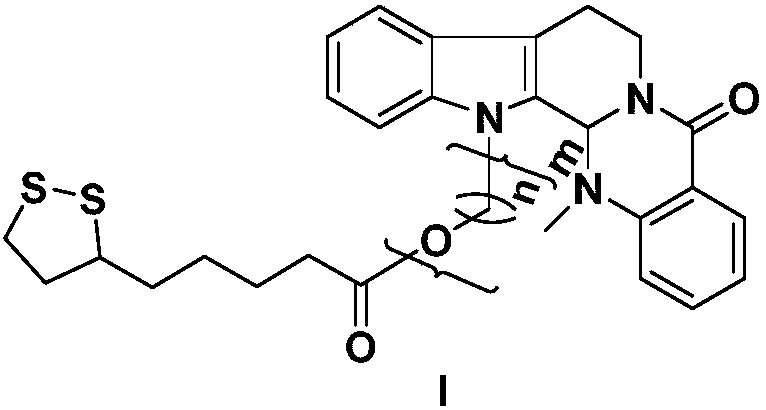
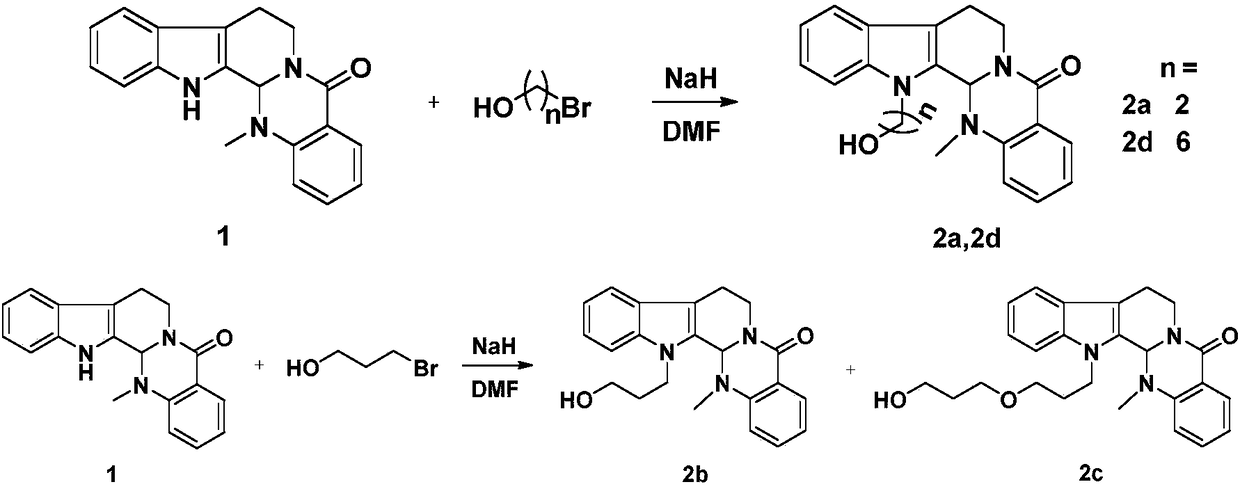
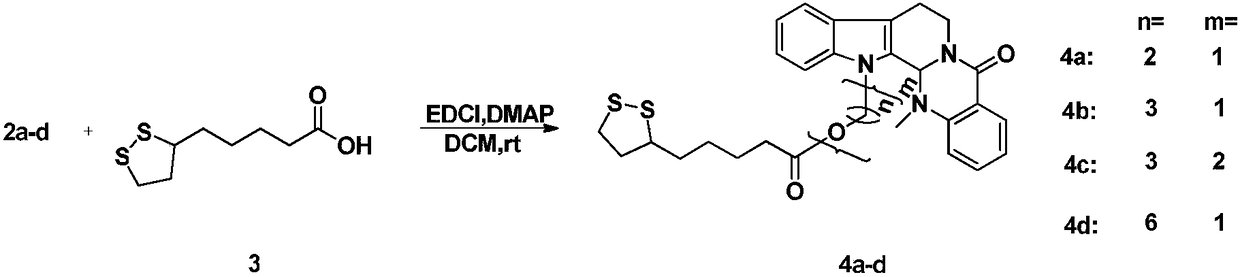
Complex of alpha-lipoic acid H2S donor and evodiamine, and preparation method and application thereof
A technology of evodiamine and donor, applied to derivatives modified at the N-13 site, applied in the preparation of anti-tumor drugs, can solve the problems of unsuitable release control, high toxicity, and inability to be directly applied to clinical research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023]
[0024] Take evodiamine intermediate 2a (40mg, 0.12mmol), dissolve it in dichloromethane (5mL), add α-lipoic acid (30mg, 0.15mmol), EDCI (62mg, 0.30mmol), DMAP (4mg, 0.02mmol) ), the reaction was stirred at room temperature, the reaction progress was monitored by TCL, and the reaction was terminated after 12 hours. The reaction solution was poured into 10ml of ice-water mixture, extracted with dichloromethane (10mL×3), washed with saturated saline solution, dried over anhydrous sodium sulfate, recovered dichloromethane to obtain crude product 4a, passed through a silica gel column (petroleum ether: acetic acid Ethyl ester=10:1), separated to obtain a light yellow solid with a yield of 55%. HRMS(ESI)m / z calcd for C 29 h 33 N 3 HO 3 S 2 [M+H] + 536.2036, found 536.2057. 1 H NMR (CDCl 3 ,400MHz), δ(ppm):8.15(dd,J=7.8,1.2Hz,1H,Ar-H),7.61(d,J=7.8Hz,1H,Ar-H),7.51(td,J=7.9 ,1.2Hz,1H,Ar-H),7.45(d,J=7.9Hz,1H,Ar-H),7.31(d,J=7.2Hz,1H,Ar-H),7.28(m,1H,Ar -H),7.22(m,1H,...
Embodiment 2
[0026]
[0027] Compound 4b was prepared according to the synthesis method of Example 1. Pale yellow solid, yield 66%. HRMS(ESI)m / z:calcd for C 30 h 35 N 3 HO 3 S 2 [M+H] + 550.2193,found 550.2176. 1 H NMR (CDCl 3 ,600MHz), δ(ppm):8.14(dd,J=7.8,1.4Hz,1H,Ar-H),7.61(d,J=7.8Hz,1H,Ar-H),7.51(m,1H,Ar-H) -H),7.38(m,1H,Ar-H),7.30(m,1H,Ar-H),7.25(m,1H,Ar-H),7.21(m,1H,Ar-H),7.18( m,1H,Ar-H),5.98(s,1H,N-CH-N),4.91(m,1H,N-CH 2 ),4.54(m,1H,13-N-CH 2 ),4.28(m,1H,13-N-CH 2 ),4.17(m,1H,-COOCH 2 ),4.03(m,1H,-COOCH 2 ),3.54(m,1H,N-CH 2 ),3.20(m,2H,-CH 2 ),3.11(m,1H,-CH 2 ),3.03(m,1H,-CH 2 ),2.89(m,1H,-CH 2 ),2.45(m,1H,-CH 2 ),2.40(s,3H,N-CH 3 ),2.18(m,2H,-CH 2 ),2.12(m,2H,-CH 2),1.89(m,1H,-S-CH-),1.34–1.65(m,6H,-CH 2 ). 13 C NMR (CDCl 3 ,150MHz)δ(ppm):173.32,164.62,150.98,137.17,133.02,129.07,128.41,125.93,124.47,124.26,123.23,122.88,119.86,119.24,113.45,109.61,68.02,61.61,56.45,40.70,40.35, 39.37, 38.60, 36.48, 34.61, 33.92, 29.25, 28.82, 24.58, 20.42.
Embodiment 3
[0029]
[0030] Compound 4c was prepared according to the synthesis method of Example 1. Pale yellow solid, yield 65%. HR-MS(ESI)m / z:calcd for C 33 h 41 N 3 NaO 4 S 2 [M+Na] + 630.2431, found 630.2501. 1 H NMR (CDCl 3 ,600MHz), δ(ppm):8.13(dd,J=7.8,1.4Hz,1H,Ar-H),7.61(d,J=7.8Hz,1H,Ar-H),7.49(m,1H,Ar-H) -H),7.43(m,1H,Ar-H),7.28(m,1H,Ar-H),7.23(m,1H,Ar-H),7.20(m,1H,Ar-H),7.17( m,1H,Ar-H),6.00(s,1H,N-CH-N),4.90(m,1H,N-CH 2 ),4.56(m,1H,13-N-CH 2 ),4.28(m,1H,13-N-CH 2 ),4.08(m,1H,-COOCH 2 ),4.03(m,1H,-COOCH 2 ),3.55(m,1H,N-CH 2 ),3.42(m,1H,-CH 2 -O-CH 2 ),3.37(m,2H,-CH 2 -O-CH 2 ),3.34(m,1H,-CH 2 -O-CH 2 ),3.18(m,2H,-CH 2 ),3.10(m,1H,-CH 2 ),3.03(m,1H,-CH 2 ),2.89(m,1H,-CH 2 ),2.45(m,1H,-CH 2 ),2.40(s,3H,N-CH 3 ),2.30(m,2H,-CH 2 ),2.06(m,2H,-CH 2 ), 1.89(m,1H,-S-CH-),1.62–1.77(m,6H,-CH 2 ),1.47(m,2H,-CH 2 ). 13 CNMR (CDCl 3 ,150MHz)δ(ppm):173.51,164.69,151.02,137.35,132.94,128.98,128.62,125.79,124.18,124.13,123.13,122.68,119.66,119.05,113.16,109.9...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap