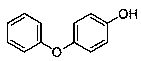
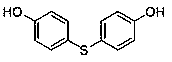
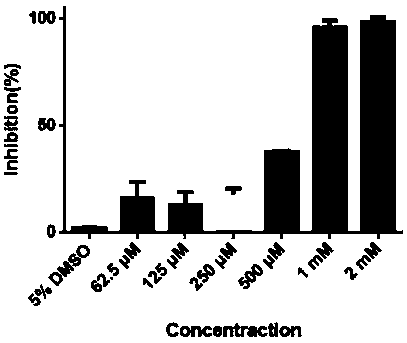
Medicine binding pocket of DNA (deoxyribonucleic acid) gyrase and application thereof
A technology of gyrase and drugs, applied in the field of biomedicine, to achieve the effect of avoiding drug resistance problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] 1. Construction of the prokaryotic expression plasmid of the E. coli DNA gyrase GyrB subunit ATPase domain
[0058] Insert the DNA coding sequence of the ATPase domain (amino acid sequence 15-220) of the Escherichia coli DNA gyrase GyrB subunit (UniProKB numbering P0AES6) into the pET20b (+) vector, and insert six sets of upstream of the DNA coding sequence Amino acid tag and yeast sumo protein tag DNA sequence, constructed to express His 6 -Prokaryotic expression plasmid of sumo-GyrB fusion protein. The inserted DNA sequence was verified by sequencing.
[0059] The amino acid sequence of the Escherichia coli DNA gyrase GyrB subunit ATPase domain (amino acid sequence 15-220) is:
[0060] GGLDAVRKRPGMYIGDTDDGTGLHHMVFEVVDNAIDEALAGHCKEIIVTIHADNSVSVQDDGRGIPTGIHPEEGVSAAEVIMTVLHAGGKFDDNSYKVSGGLHGVGVSVVNALSQKLELVIQREGKIHRQIYEHGVPQAPLAVTGETEKTGTMRFWPSLETFTNVTEFEYEILAKRLRELSRDFLNSGKVSIRL
[0061] The DNA sequence encoding the Escherichia coli DNA gyrase GyrB subunit ATPase do...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Half inhibitory concentration | aaaaa | aaaaa |
| Half inhibitory concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com