Application of reserpine in preparing anti-porcine epidemic diarrhea virus medicine
A technology for porcine epidemic diarrhea and reserpine, which is applied in antiviral agents, pharmaceutical formulations, organic active ingredients, etc., can solve the problem that virus particles do not have hemagglutination, and achieve the effects of easy absorption, low drug, and significant effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
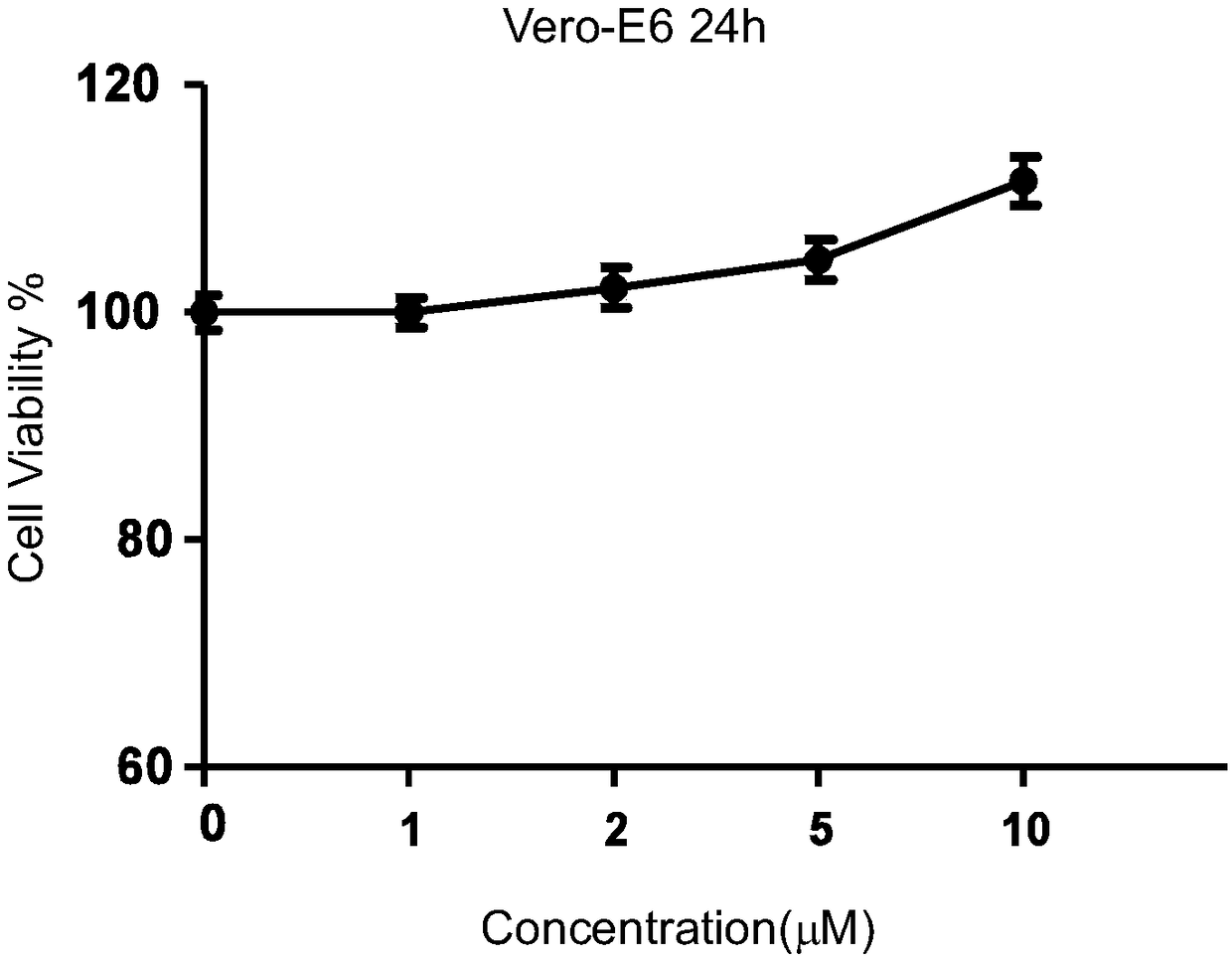
[0027] Example 1 Reserpine Cytotoxicity Determination on Vero-E6 Cells
[0028] After counting Vero-E6 cells, they were diluted with 8% fetal bovine serum (FBS) DMEM nutrient solution to an appropriate density and then diluted to 1.5×10 4 The concentration per well was added to a 96-well plate and placed at 37°C, 5% CO 2 After the cells adhered to form a monolayer in the incubator (about 18-20h), the reserpine was diluted with DMEM nutrient solution to: 1 μM, 2 μM, 5 μM, 10 μM, and three sets of repetitions were set for each concentration. After treating Vero-E6 cells with 100 μl / well of DMSO control and diluted reserpine samples on a 96-well cell culture plate for 72 hours, remove the supernatant, add 90 μl of fresh culture medium, and then add 10 μl of MTT solution, and continue to incubate for 4 hours; Remove the supernatant, add 110 μl Formazan solution to each well, place on a shaker and shake at low speed for 10 minutes to fully dissolve the crystals. Measure the abs...
Embodiment 2
[0030] Example 2 Western Blot Determination of Reserpine Inhibiting Porcine Epidemic Diarrhea Virus Infection on Vero-E6 Cells Activity
[0031] After Vero-E6 cells were counted, they were diluted with 8% fetal bovine serum (FBS) DMEM nutrient solution to an appropriate density and then diluted to 7×10 5 The concentration per well was added to a 6-well plate and placed at 37°C, 5% CO 2 After the cells adhered to form a monolayer in the incubator and grew to a density of 70-80% (about 18-20 hours), the reserpine was diluted with DMEM nutrient solution to: 1 μM, 2 μM, 5 μM, 10 μM. DMSO control and different concentrations of reserpine were pre-treated Vero-E6 cells at 37°C for 2 hours, then infected with porcine epidemic diarrhea virus HLJBY strain (MOI=0.01), and placed at 37°C, 5% CO 2 Place in the incubator for 1 hour, wash three times with PBS, add 1ml of DMEM maintenance solution containing 2% fetal bovine serum (FBS) and contain reserpine, place at 37°C, 5% CO 2 Afte...
Embodiment 3
[0032] Example 3 Fluorescent quantitative detection of the activity of reserpine in inhibiting porcine epidemic diarrhea virus infection on Vero-E6 cells sex
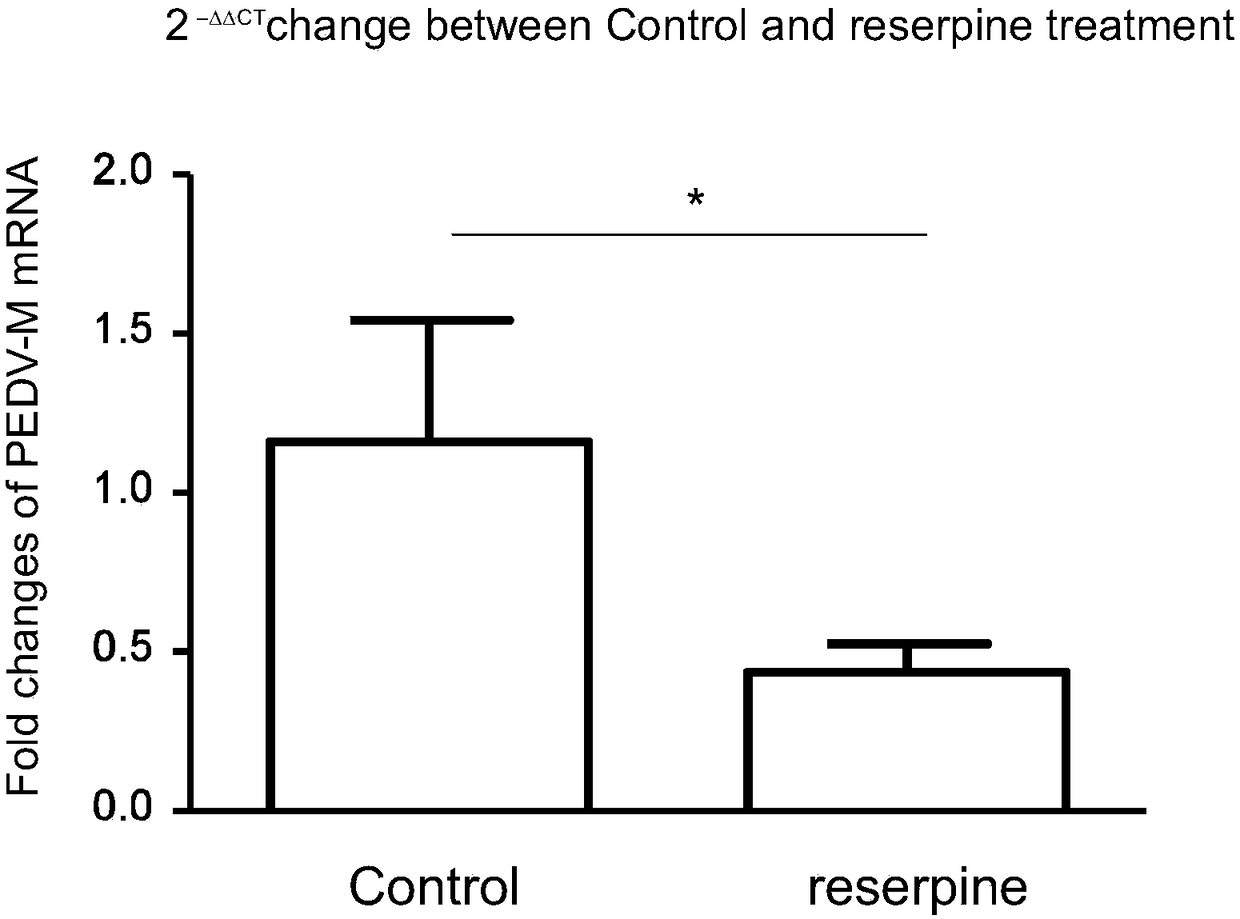
[0033] After Vero-E6 cells were counted, they were diluted with 8% fetal bovine serum (FBS) DMEM nutrient solution to an appropriate density and then diluted to 7×10 5 The concentration per well was added to a 6-well plate and placed at 37°C, 5% CO 2After the cells adhered to form a monolayer in the incubator and grew to a density of 70-80% (about 18-20 hours), the reserpine was diluted to 10 μM with DMEM nutrient solution. After pre-treating Vero-E6 cells with DMSO control and reserpine at 37°C for 2 hours, they were infected with porcine epidemic diarrhea virus HLJBY strain (MOI=0.01), and placed at 37°C, 5% CO 2 Place in the incubator for 1 hour, wash three times with PBS, add 1ml of DMEM maintenance solution containing 2% fetal bovine serum (FBS) and contain reserpine, place at 37°C, 5% CO 2 After culturing in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com