Chlorine dioxide solution used for oral administration
A chlorine dioxide and solution technology, applied in the digestive system, chlorine active ingredients, dispersion liquid delivery, etc., can solve problems such as poor cure rate, health hazards, and normal intestinal flora imbalance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0052] The preparation method of the modified diatomite loaded with chlorine dioxide comprises the following steps:
[0053] (1) Calcinate the diatomite in a muffle furnace at 450°C for 2h, cool to room temperature, soak it in a 70°C water bath for 4h with a sulfuric acid solution with a mass fraction of 70%, dilute it with deionized water, filter, and washed with deionized water several times until the filtrate is neutral, dried under reduced pressure at 70°C for 24 hours, and ground to obtain the treated diatomite; the diatomite and the sulfuric acid solution with a mass fraction of 70%, the deionized The weight ratio of ionized water (for dilution) is 1:6:20;
[0054] (2) Add absolute ethanol to the diatomite treated in step (1), stir at room temperature for 1 h, then add glycerol hydroxystearate, heat up to 70°C, keep the temperature for 2h, cool down to room temperature, filter, 70°C Drying under reduced pressure for 20 hours, grinding to obtain glycerol hydroxystearate ...
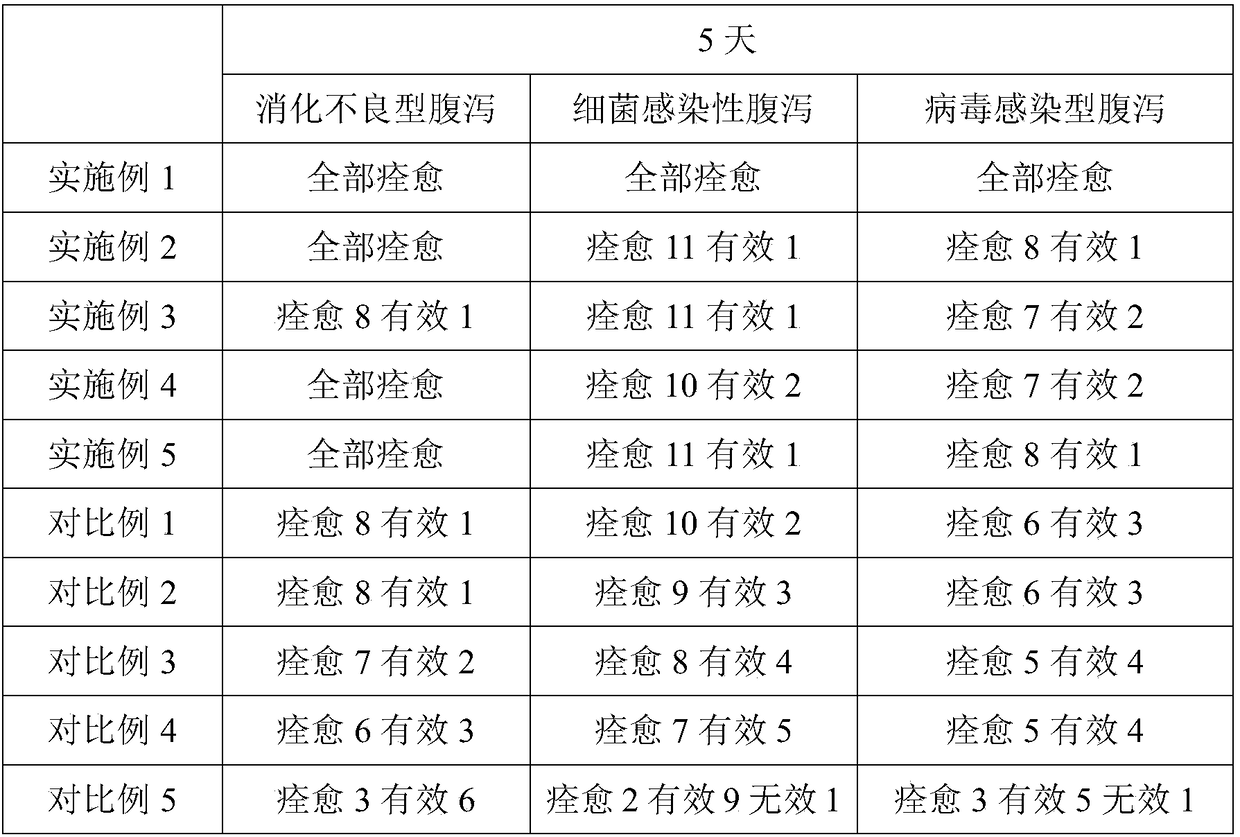
Embodiment 1
[0064] The chlorine dioxide solution for oral administration includes A component and B component, and the A component includes at least Scutellaria baicalensis extract, Forsythia extract, Dandelion extract, Cortex Phellodendri extract, Atractylodes macrocephala extract, Cortex Moutan extract, Poria cocos extract, plantago seed extract, ephedra extract, yam extract, Codonopsis pilosula extract, licorice extract, cinnamon extract; the B component includes modified diatomaceous earth loaded with chlorine dioxide , deionized water; the weight ratio of the A component to the B component is 1:16; the weight ratio of the modified diatomite loaded with chlorine dioxide in the B component to the deionized water is 1:45; the preparation raw material of modified diatomite in the modified diatomite loaded with chlorine dioxide at least includes diatomite, glycerol hydroxystearate, chitosan-guanidine salt complex; The weight ratio of the diatomite to the glycerol hydroxystearate and the c...
Embodiment 2
[0074] The chlorine dioxide solution for oral administration includes A component and B component, and the A component includes at least Scutellaria baicalensis extract, Forsythia extract, Dandelion extract, Cortex Phellodendri extract, Atractylodes macrocephala extract, Cortex Moutan extract, Poria cocos extract, plantago seed extract, ephedra extract, yam extract, Codonopsis pilosula extract, licorice extract, cinnamon extract; the B component includes modified diatomaceous earth loaded with chlorine dioxide , deionized water; the weight ratio of the A component to the B component is 1:10; the weight ratio of the modified diatomite loaded with chlorine dioxide in the B component to the deionized water is 1:45; the preparation raw material of modified diatomite in the modified diatomite loaded with chlorine dioxide at least includes diatomite, glycerol hydroxystearate, chitosan-guanidine salt complex; The weight ratio of the diatomite to the glycerol hydroxystearate and the c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com