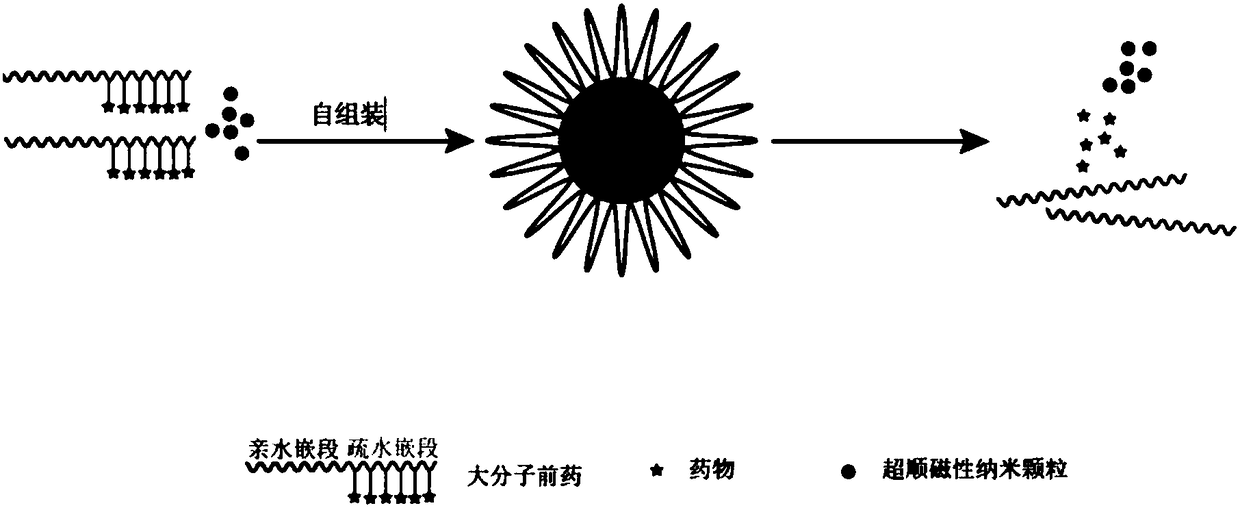
Composite prodrug nanocarrier for reversing tumor drug resistance and preparation method thereof
A nano-carrier and drug-resistant technology, applied in the fields of polymer materials and medical engineering, can solve the risk of weakening the synchronization of hyperthermia and chemotherapy, increasing the side effects of chemotherapy drugs, and high non-targeted hyperthermia and chemotherapy synergy and other issues, to achieve the effect of reversing tumor drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Weigh 100mg of polyhydroxypropyl methacrylate-polyethylene glycol copolymer, and react it with 20mg of carboxylated tetravalent platinum to obtain a macromolecular prodrug linked to tetravalent platinum through an ester bond, poly(methyl Hydroxypropyl acrylate-quaternary platinum)-polyethylene glycol, wherein the coupling rate of polyhydroxypropyl methacrylate and carboxylated tetravalent platinum is 90%.
[0029] Take 5mg of particle size as 8nm superparamagnetic nanoparticles MnFe 2 o 4 and 5mg of platinum macromolecule prodrug poly(hydroxypropyl methacrylate-tetravalent platinum)-polyethylene glycol, dispersed in 2mL of tetrahydrofuran / NN-dimethylformamide=1 / 1 mixed solution, and then The mixed solution was added dropwise to 20 times the volume of deionized water under the action of ultrasound, and then dialyzed in deionized water for 24 hours to remove the organic solvent to obtain the aqueous solution of the composite prodrug nanocarrier. The composite prodrug na...
Embodiment 2
[0031] Weigh 80 mg of poly-N-methylolacrylamide-polyethylene glycol copolymer, modify the hydroxyl group of poly-N-methylolacrylamide into hydrazine, and then react with 20 mg of doxorubicin hydrochloride to obtain Adriamycin-linked macromolecule prodrug, poly(N-hydroxyethyl methacrylate-doxorubicin)-polyethylene glycol, wherein the modified poly N-hydroxyethyl methacrylate and doxorubicin The connection rate is 86%.
[0032] Get 2mg of particle diameter and be the superparamagnetic nanoparticle Mn of 15nm 0.6 Zn 0.4 Fe 2 o 4 And 5mg of macromolecular prodrug poly(N-methylolacrylamide-doxorubicin)-polyethylene glycol, add in the mixed solution of 5mL tetrahydrofuran / dimethyl sulfoxide=1 / 1, then in ultrasonic The mixed solution was added dropwise to 10 times the volume of deionized water, and then dialyzed in deionized water for 24 hours to remove the organic solvent to obtain an aqueous solution of the composite prodrug nanocarrier. The composite prodrug nanocarrier was t...
Embodiment 3
[0034] Take by weighing 50mg of polyhydroxyethyl methacrylate-polyethylene glycol copolymer, react it with the methotrexate of 10mg, obtain the macromolecule prodrug that is connected with methotrexate by ester bond, poly(methyl Hydroxypropyl acrylate-methotrexate)-polyethylene glycol, wherein the coupling rate of polyhydroxyethyl methacrylate and methotrexate is 88%.
[0035] Get 2mg of particle diameter and be the superparamagnetic nanoparticle Mn of 12nm 0.4 Zn 0.6 Fe 2 o 4 And 4mg of macromolecule prodrug poly(hydroxypropyl methacrylate-methotrexate)-polyethylene glycol, add 4mL of tetrahydrofuran / NN-dimethylformamide=3 / 7 in the mixed solution, then in Under the action of ultrasound, the mixed solution was added dropwise to 20 times the volume of deionized water, and then dialyzed in deionized water for 24 hours to remove the organic solvent to obtain an aqueous solution of the composite prodrug nanocarrier. The composite prodrug nanocarrier was then lyophilized to obt...
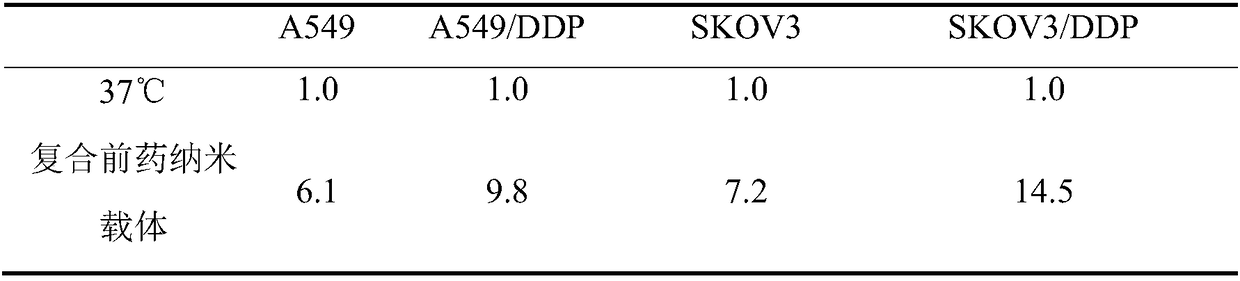
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com