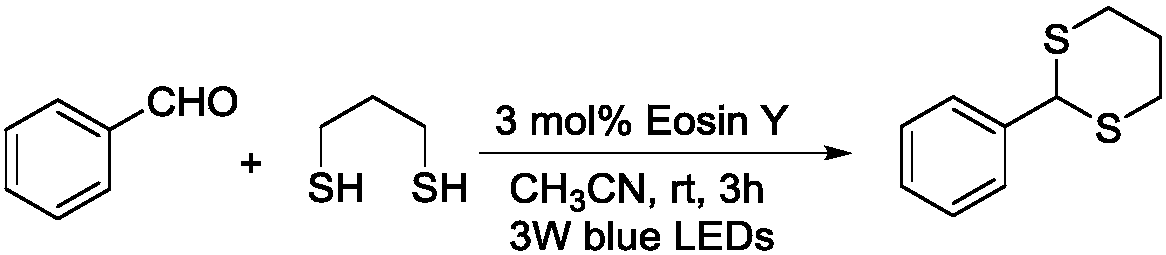Preparing method of 2-substituted-1,3-dithiane derivative
A technology of derivatives and dithianes, which is applied in the field of preparation of 2-substituted-1,3-dithiane derivatives, achieves the effects of high substrate conversion rate, simple and easy post-treatment, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
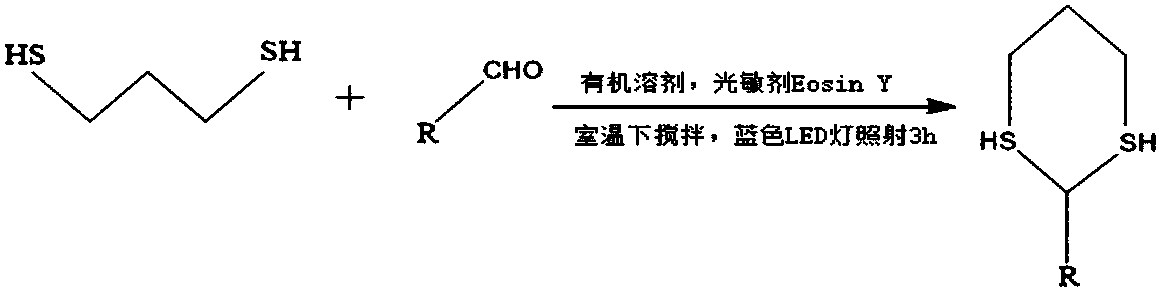
[0021] The synthetic method of 2-phenyl substituted-1,3-dithiane comprises the following steps:
[0022] (1) Add 0.55mmol of 1,3-propanedithiol, 0.5mmol of benzaldehyde and 0.015mmol of Eosin Y to a 10mL colorless transparent glass test tube, add 1.5mL of MeCN to dissolve, and stir the system at room temperature 30°C , with a 3w blue LED light for 3h.
[0023] (2) After the reaction was completed, MeCN was removed by rotary evaporation under reduced pressure and the resulting mixture was separated by silica gel column chromatography to obtain the product.
[0024] The specific synthetic reaction formula is:
[0025]
[0026] After detection, the characterization result of the obtained 2-phenyl substituted-1,3-dithiane is: the yield is 98%, white solid. 1 H NMR (400MHz, CDCl 3 )δ7.53–7.41(m,2H),7.40–7.28(m,3H),5.17(s,1H),3.16–2.98(m,2H),3.00–2.83(m,2H),2.27–2.08( m,1H),2.05–1.82(m,1H); 13 C NMR (101MHz, CDCl 3 )δ 139.0, 128.7, 128.4, 127.7, 51.4, 32.1, 25.0.
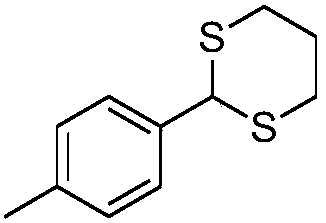
Embodiment 2
[0028]
[0029] The product is as shown above, and the reaction conditions and steps are the same as in Example 1. After testing, the characterization result of the obtained product is: the yield is 99%, white solid. 1 H NMR (400MHz, CDCl3) δ7.33 (d, J = 8.0Hz, 2H), 7.13 (d, J = 8.0Hz, 2H), 5.13 (s, 1H), 3.05–2.98 (m, 2H), 2.88 –2.83(m,2H),2.31(s,3H),2.14–2.09(m,1H),1.86–1.82(m,1H); 13 C NMR (101 MHz, CDCl3) δ 138.1, 136.0, 129.2, 127.4, 51.0, 32.0, 24.9, 21.1.
Embodiment 3
[0031]
[0032] The product is as shown above, and the reaction conditions and steps are the same as in Example 1. After detection, the characterization result of the obtained product is: the yield is 74%, white solid. 1 H NMR (400MHz, CDCl3) δ7.47 (d, J = 8.4Hz, 2H), 7.35 (d, J = 8.4Hz, 2H), 5.11 (s, 1H), 3.15–2.97 (m, 2H), 2.93 –2.88(m,2H),2.20–2.14(m,1H),1.96–1.87(m,1H); 13 CNMR (101MHz, CDCl3) δ138.0, 131.9, 129.5, 122.3, 50.6, 31.9, 24.9.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com